Depression is a common mental disorder, that affects more than 350 million people all around the world and it could be second most common disease after ischaemic heart disease in the world by the year 2020 [1,2]. In India about one in five people are affected by depressive disorders [3]. Depression in the early stages induces various adaptational physiologic responses. Chronic depression might lead to hyperactivity of Hypothalamus-Pituitary-Adrenal (HPA) axis as well as Sympathetic-Adrenal-Medullary (SAM) system and make them more vulnerable to various diseases like hypertension, diabetes, cardiovascular diseases and salivary gland dysfunction [4,5]. Salivary gland function is controlled by both sympathetic and parasympathetic innervations and in depression, both sympathetic and parasympathetic system get affected which leads to altered salivary function, this could reflect in altered salivary composition also [5-7].
Antidepressants are the drugs which elevate the mood in depressive disorders and may act on salivary gland neural controls or on the glandular cells resulting in hyposalivation: besides from influencing the amount of saliva, they may also alter the composition of the saliva [8,9]. Salivary gland dysfunction may alter the salivary composition which may cause transient inconvenience to severe impairments of oral health such as mucositis, burning sensation, altered taste sensation, dental caries, periodontal diseases and oral candidiasis [10,11].
Sialochemistry, an emerging diagnostic tool that can gives qualitative data on certain imperative parameters of salivation is utilised for diagnosis and research purpose [12]. Previous studies showed both depression and antidepressant drugs could altered salivary secretion and composition [12-16]. However, there was a lacunae in analysing sialochemical alteration, the pre and post medication status, of antidepressant therapy. So, the aim of the present study was to find out significant sialochemical alteration in unstimulated saliva of depressive individuals and to compare the effect of antidepressant therapy SSRI two month course on salivary composition in the same patients.
Materials and Methods
This observational study was conducted in the Department of Psychiatry, Mahatma Gandhi Medical college and Research Institute, Puducherry, India, over a period of six months between January to June 2016. The study proposal was approved by Institutional Review Board (IRB) and Institutional Ethical Committee (IEC).
The sample size was calculated by using the following formula:
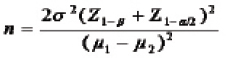
Where, n=required sample size, Z-z score or standard score, Z1-α/2=1.96 (confidence level at 95%) and Z1-β/2=1.28, with significance level α=0.05, power=90%, Mean (μ1)=28.15 and (μ2)=15.94.
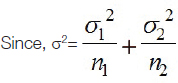
Standard deviation (σ1)=22.42 and (σ2)=1.11 n1 and n2=50.
{Mean (μ), Standard deviation (σ) and n1 and n2 values were obtained from key article} [16].
The (n=36), with drop-out rate of 10% the sample size increased to 40. Hence, the sample size was rounded off to 40 subjects per group.
A total of 80 subjects between the age range of 18-50 years were included in the study.
Patient Selection Criteria
Suspected depressive patients were thoroughly examined by the clinical psychiatrist. Based on the depression and anxiety assessment by using, HADS (developed by Zigmond AS and Snaith RP) and clinical history, patients were diagnosed as depressive and non-depressive individuals [17]. Subjects with systemic disorders such as diabetic mellitus, hypertension, autoimmune disorders etc., or under any other medication that alters salivary secretion, or radio/chemotherapy in head and neck region in the last six months, or depressive patients under any other antidepressant therapy other than SSRI class of drug were excluded from the study. Study groups were categorised as follows:
Group I: Control subjects (non-depressive patients with HADS value D≤8 and A≤8) (40 samples).
Group II: Depressive patients not under medication (with HADS value D≥8 and A≥8) (40 samples).
Group III: Group II patients who have been on SSRI antidepressants for two months. These 40 samples were the subjects in Group II who were given medication.
Sample Collection and Processing
Unstimulated whole salivary samples were collected between 8 am-12 pm (to avoid circadian variations), based on spitting method illustrated by Navazesh M, spitting method [18]. The individuals were instructed to abstain from eating, drinking (with the exception of water), tooth brushing, practice physical exercises or be under great physical stress for at least one hour prior to sample collection. The subjects were told to wash their mouths completely with deionised water and allowed to sit in a relaxed position for five minutes and allow saliva to aggregate in the mouth. They were instructed to expectorate into a sterile plastic container usually once every 60 seconds over a period of five minutes [11,18]. Then the collected sample was taken to the Biochemistry laboratory. Salivary samples were centrifuged at 3200 rpm for 10 minutes and supernatant fluid was collected and subjected to sialochemical analysis. Salivary potassium, sodium, and chloride levels were estimated by Ion selective electrode method, Easylyte analyser (Medica). Spectrophotometric analysis of salivary α-amylase, total protein, urea, calcium was done by International Federation of Clinical Chemistry (IFCC) approved method, using a Hitachi 902 autoanalyser [11,16].
Statistical Analysis
The analysis was carried out using SPSS version 21.0. Mean and Standard Deviation (SD) were used for describing the data. Unpaired t-test was used to compare the salivary parameters between the Groups I and II and Paired t-test was used to compare the salivary parameters between the Group II and III.
Results
The following observations were tabulated [Table/Fig-1,2] along with statistical analysis and [Table/Fig-3] showed comparison of mean value study parameters among Group I, II and III.
Shows comparison of statistical values among Group I and Group II.
| Salivary parameters (variables) | n | Mean±SD | 95% Confidence interval | t-value | df | p-value |
|---|
| Group I | Group II | Lower | Upper |
|---|
| Salivary pH | 40 | 6.39±0.39 | 6.46±0.44 | -0.1115 | 0.2555 | 0.781 | 78 | 0.44 |
| Sodium (mEq/L) | 40 | 11.23±4.92 | 13.40±6.35 | -0.3569 | 4.7039 | 1.710 | 78 | 0.09 |
| Potassium (mEq/L) | 40 | 23.84±5.37 | 25.30±6.48 | -1.188 | 4.1116 | 1.098 | 78 | 0.28 |
| Chloride (mEq/L) | 40 | 20.17±5.80 | 24.30±9.81 | 0.529 | 7.726 | 2.292 | 63.28 | 0.03* |
| Total protein (gm/dL) | 40 | 0.24±0.12 | 0.55±0.32 | 0.2058 | 0.42420 | 5.796 | 49.11 | 0.0001* |
| Salivary α-amylase (U/L) | 40 | 39.29±23.44 | 61.13±56.02 | 2.574 | 41.1111 | 2.274 | 52.25 | 0.03* |
| Urea (mg/dL) | 40 | 27.43±9.67 | 28.31±14.9 | 2.814 | -4.718 | 0.314 | 78 | 0.75 |
| Calcium (mg/dL) | 40 | 5.39±1.92 | 6.21±2.24 | -0.1114 | 1.7519 | 1.753 | 78 | 0.08 |
Unpaired t-test, n: Total no. of sample per group, SD: Standard deviation, S: Significant, df: Degrees of freedom, *-Significant p-value
Shows comparison of statistical values among Group II and Group III.
| Salivary parameters (variables) | n | Mean±SD | 95% Confidence interval | t-value | df | p-value |
|---|
| Group II | Group III | Lower | Upper |
|---|
| Salivary pH | 40 | 6.46±0.44 | 6.58±0.47 | -0.3343 | 0.1018 | -1.079 | 39 | 0.29 |
| Sodium (mEq/L) | 40 | 13.40±6.35 | 13.09±4.31 | -2.2651 | 2.8891 | 0.245 | 39 | 0.081 |
| Potassium (mEq/L) | 40 | 25.30±6.48 | 24.95±5.43 | -2.0944 | 2.7989 | 0.291 | 39 | 0.77 |
| Chloride (mEq/L) | 40 | 24.30±9.81 | 21.24±6.56 | -0.402 | 6.515 | 1.788 | 39 | 0.08 |
| Total protein (gm/dL) | 40 | 0.55±0.32 | 0.38±0.32 | 0.02595 | 0.31055 | 2.392 | 39 | 0.02* |
| Salivary α-amylase (U/L) | 40 | 61.13±56.02 | 49.54±28.52 | -9.2135 | 32.3835 | 1.127 | 39 | 0.27 |
| Urea (mg/dL) | 40 | 28.31±14.94 | 25.81±8.07 | -3.3403 | 8.3553 | 0.867 | 39 | 0.39 |
| Calcium (mg/dL) | 40 | 6.21±2.24 | 5.31±1.39 | 0. 17716 | 1.61334 | 2.522 | 39 | 0.02* |
Paired t-test, n: Total no. of sample per group, SD: Standard deviation, S: Significant, df: Degrees of freedom, *-Significant p-value
Showing comparison of mean value study parameters among Group I, II and III.
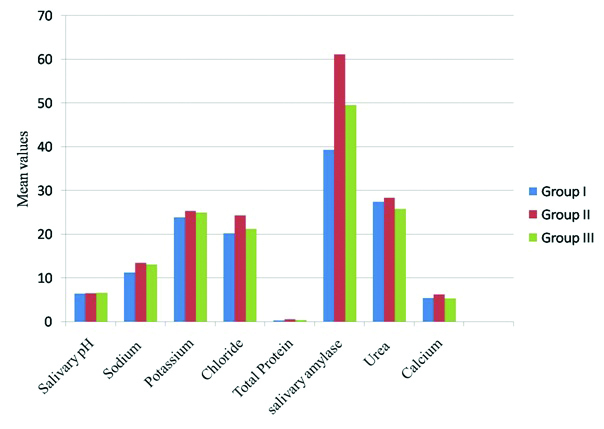
Depressive individuals not under medication (Group II) showed a statistically significant sialochemical alteration in certain parameters like total protein, α-amylase, chloride levels and did not show any significant sialochemical alteration in sodium, potassium, calcium, urea and salivary pH when compared to healthy controls (Group I) [Table/Fig-1].
Depressive individuals under SSRI (Group III) showed a statistically significant decrease in total protein level and calcium level and there was no statistically significant alteration in salivary pH, sodium, potassium, chloride, α-amylase and urea levels compared to depressive individuals not under medication (Group II) [Table/Fig-2].
Discussion
Sialochemistry is a useful means of chronologically monitoring quantitative changes of chemicals that are present in saliva. The present study analysed the sialochemical alteration of depressive individuals and compared the effect of SSRI on the same patients. In the present study, SSRI was preferred over other drugs as it has less systemic side effects [11].
Post-therapeutic samples were collected after two months duration of treatment. This was done based on the fact that the turnover rate of salivary gland cells is 60-120 days [19]. This is the time period required for an individual to present with any change in the quantity and quality of saliva profile.
The age range of 18-50 years was selected to avoid variations in salivary gland secretion since it is found to be more active in younger individuals and least active in elderly individuals [20,21]. Systemic conditions that could affect the quality and quantity of saliva were excluded from the study [12,20].
The parameters chosen for the present study was based on presumed relationship with intraglandular transport processes such as sodium, potassium, chloride; intracellular synthesis such as protein, α-amylase and diffusion of plasma constituents such as urea [22].
The present study analysed the salivary total protein and salivary α-amylase level in depressive individuals (Group II) and found a statistically significant increase in total protein (p=0.0001*), and salivary α-amylase (p=0.03*) concentration when compared with the non-depressive individuals (Group I). The result of the raised salivary amylase in depression is consistent with findings of Booij SH et al., and Jung JY et al., [23,24]. Vineetha R et al., revealed that increase in salivary α-amylase level during psychosocial stress might be explained by physiological response to stress [5].
Scannapieco FA et al., showed that raised level of salivary amylase promoted adhesion of oral Streptococci and may have role in dental plaque and caries formation [25]. Few other studies state that salivary total protein concentration is increased in gingivitis and periodontitis [26]. Deshpande RR et al., in their study reviewed that the salivary proteins can be considered as “double edge sword” since it has both protective and detrimental roles. The function may depend on the molecule’s location or site of action, i.e., antimicrobial and pH modulating proteins play a protective role; while agglutinin plays a detrimental role in colonisation of microorganisms [27].
There was a statistically significant decrease in the total protein (p=0.02) and slight decrease in salivary α-amylase level (p=0.27) in patients after two months of antidepressant therapy. In the present study, SSRI was the prescribed drug, since it does not have much effect on salivary gland secretion due to absence of serotonin receptor [28]. Hence, decrease in total protein and salivary α-amylase might be due to the reestablishment of homeostasis by SSRI. de Almeida PV et al., Veen G et al., and Milton BA et al., in their studies compared the sialochemical alteration of various antidepressant drugs like Tricyclic Antidepressant (TCA), TeCA and SSRI and found that SSRI did not show much significant sialochemical alteration [14-16].
The important factors affecting the composition of saliva are flow rate, differential gland contribution, circadian rhythm, duration and nature of stimulus and medication. At high flow rate salivary Bicarbonate (HCO3-) concentration increases, Sodium/Hydrogen (Na+/H+) exchange serves to restore the intracellular pH and positively correlated with salivary flow rate, whereas, the concentration of potassium and chloride were negatively correlated. The low flow rate produces significant electrolyte alteration with decreased salivary pH [29]. Morse DR et al., Sandin B and Chorot P, Khalaila R et al., and Cohen M et al., reported decreased salivary pH in stressed, anxiety and depressed patients [30-33]. The present study did not show any significant change in salivary pH in any of the study groups and the values were within the normal range of 6.7 to 7.4 [29].
Major ions like sodium, potassium, chloride, calcium and bicarbonate are few of the important contributors of the osmolarity of saliva. Calcium and phosphate neutralise acid that would otherwise cause tooth demineralisation. The present study shows slight increase in calcium level in depressive individuals when compared to the non-depressive subjects but the increase was not statistically significant (p=0.08). It is also proposed that in decreased flow rate, the ductal cells may undergo more reabsorption of Na+ and makes the final secretion with less Na+ concentration. There is diminished membrane transport in depressive individuals because of which electrolyte concentration may be increased. Urea is another buffer which is a product of amino acid and protein catabolism. They increase the salivary pH by releasing ammonia and carbon dioxide when hydrolyses by bacterial urease. Increase in urea decreases the caries incidence but ammonia is potentially cytotoxic to gingival tissues and initiates periodontal diseases [34].
The present study revealed that the depressive individuals showed increase in chloride, sodium, potassium and urea levels when compared with normal individuals but the increase was statistically significant only for chloride (p=0.03) and not for other parameters. Brown in 1970 supported the view that manic depressive patients secrete saliva slightly lower than normal and in depressive phase they secrete much less saliva [15]. Glen AI et al., found that there was significant increase in sodium level in manic-depressive or recurrent depressive illness in parotid flow rate [13]. In contrast to this, no specific alteration in electrolyte pattern was reported by Bolwig TG and Rafaelsen OJ, in manic-depressive patients in mixed saliva and also suggested that, the mixed saliva would preclude the modest difference among the major and minor salivary glands [35].
Milton BA et al., analysed the electrolyte alteration and revealed a statistically significant increase in urea and calcium and decrease in sodium level in depressed individuals and depressed individuals under medication when compared with normal individuals. They also stated that TCA produced significant sialochemical alteration and SSRI did not cause much sialochemical variation [16], this was also supported by de Almeida PV et al., and Hunter KD and Wilson WS [14,36]. They also concluded that, the deleterious effects of SSRI is less when compared to TCA, however, even SSRI produce decreases in salivary flow rate with oral manifestation of xerostomia. This sialochemical alteration is probably due to relative lack of affinity of muscarinic receptor. In the present study, while comparing the depressed individuals not under medication and depressed individuals under two month course of SSRI, there was a statistically significant decrease in calcium level and total protein (p=0.02) with non statistically significant decrease in other parameters in depressive individuals under SSRI.
Limitation
The present study lacks in analysing the oral side effects due to sialochemical alteration and in gland-specific salivary alteration, hence these fields remain open for further investigation.
There are only limited studies done in this area and also most of the studies used stimulated saliva for sialochemical analysis. However further studies with large sample size and estimation of gland specific sialochemical analysis has to be initiated to add more information to the existing scientific evidence.
Conclusion
In the present study, an attempt was made to assess and bring about a comparison of sialochemical alteration among Group I, II and III. The present study concluded that Group III individuals showed significant reduction in total protein and calcium levels after two months of SSRI’s and close approximation to the levels of normal individuals. So, it means that Group III individuals got stabilised and reverted back to the normal baseline value of the healthy individuals which proves that SSRI does not produce profound sialochemical alteration.
Unpaired t-test, n: Total no. of sample per group, SD: Standard deviation, S: Significant, df: Degrees of freedom, *-Significant p-value
Paired t-test, n: Total no. of sample per group, SD: Standard deviation, S: Significant, df: Degrees of freedom, *-Significant p-value