Endometrial cancer remains to be the most common gynaecological malignancy worldwide. The disease has been classified into two main, clinically and pathologically different, molecular types: Type I is much more commonly encountered endometrioid adenocarcinoma (80–90%), and Type II comprises non endometrioid subtypes such as serous, clear cell, and undifferentiated carcinomas, as well as carcinosarcoma/malignant-mixed Müllerian tumour (10-20%), according to the ESMO-ESGO-ESTRO Consensus guidelines [1]. UPSC is a prototype of Type II EC and represents 5-10% of all EC. It is clinically aggressive and responsible for over 50% of relapses and deaths in EC patients. These tumours tend to spread outside the uterus earlier, they have high recurrence rates, and poor prognosis [2-4].
Patterns of spread for UPSC seem to be similar to ovarian cancer. Well-known risk factors for tumour spread in EC i.e., primary tumour grade, depth of invasion, and lymphovascular space invasion, have not been consistently correlated with UPSC spread. Indeed, metastatic disease can occur even in the absence of myometrial invasion and lymphovascular spread [5]. Lymph node involvement was found in up to 36% of UPSC patients with no evidence of myometrial invasion [6]. The absence of randomised controlled clinical trials for UPSC, due to its rareness, constitutes a significant problem. Therefore, the study was conducted with an aim to investigate factors affecting the overall survival in UPSC patients.
Materials and Methods
A retrospective study was performed on all women diagnosed with UPSC, treated at the Department of Gynaecologic Oncology, Izmir Katip Celebi University, Ataturk Training and Research Hospital, Turkey, between January 2006 and October 2015. Institutional Ethical Board clearance was obtained before commencement of the study. Women with a minimum of 10% component of serous carcinoma histologically were included. The cohort was limited to the patients who underwent surgical staging which included total hysterectomy, bilateral salpingo-oophorectomy, pelvic/para-aortic lymph node dissection and omentectomy. The patients without surgical treatment because of medical morbidities and those who required neoadjuvant chemotherapy were excluded from the study. Additionally, patients whose preoperative histopathological diagnosis was serous but the final histopathological diagnosis failed to meet this criterion were also excluded. Women who underwent surgical treatment with at least total hysterectomy and bilateral salpingo-oophorectomy were included.
All pathological specimens were evaluated by gynaecologic pathologists to confirm the UPSC diagnosis. Socio demographic data, systemic diseases, and all data about their disease {histopathology, tumour diameter, lymphovascular space involvement, myometrial invasion, stage, lymph node metastases (location and count), adjuvant therapies, recurrence and survival} were obtained from the hospital medical record system.
Tumour stage was retrospectively determined on the basis of surgical and pathological findings using the 2009 International Federation of Gynecology and Obstetrics (FIGO) staging system for endometrial adenocarcinoma [7]. Time elapsed between the diagnosis and death or the last follow-up was considered as the overall survival.
The factors that were analysed were the patient’s age, tumour diameter, lymphovascular space involvement, myometrial invasion, the existence of malignant cells in the peritoneal cavity, lymphatic metastasis, stage, and presence of tumour in the upper abdomen on the overall survival.
Statistical Analysis
Descriptive statistics were used to report patient demographics for all patients. Associations between categorical variables was analysed using Pearson’s chi-square and Fisher’s exact test. Survival curves by stages were generated using the Kaplan-Meier method. Univariate analysis was used to evaluate the importance of each parameter and log rank test was used for significance. Multivariate analysis was performed to identify independent predictors of survival using a Cox proportional hazards model and backwards stepwise selection. The SPSS statistical package version 22.0 was used to perform all statistical analyses (SPSS Inc., Chicago, IL, USA). The level of significance was two tailed, p<0.05.
Results
A total of 737 patients with the diagnosis of EC were treated at the Department of Gynaecologic Oncology, between January 2006 and October 2015. The initial medical record search identified 57 patients with UPSC during that period. Despite detecting UPSC in the preoperative biopsy specimens of 10 patients, none of them had UPSC in the operative specimens. Two patients who received no surgical treatment and two patients who were operated on after neoadjuvant chemotherapy were excluded from the study cohort. As a result, 43 patients with UPSC, who met all the necessary criteria were analysed.
UPSC represented 6.38% of our entire EC cohort. Median age at diagnosis was 65 years (range, 49-82 years). All patients were postmenopausal.
All patients underwent total hysterectomy and bilateral salpingo-oophorectomy, 38 (88.4%) patients were subjected to surgical staging. Omentectomy was performed in 28 patients, while 34 underwent pelvic lymph node dissection and 23 also had para-aortic lymph node dissection. Seven patients underwent more advanced cytoreductive surgery. Mean number of the removed lymph nodes was 29.91±20.42 (range: 2-86), 23.71±14.40 in the pelvic area (range: 1-58) and 8.61±7.57 in the para-aortic area (range: 1-28).
In the present study cohort, 26 of the 43 (60.5%) patients had serous tumour as the real initial diagnosis in their preoperative biopsy specimens.
In 19 (44.2%) cases the tumour infiltrated <50% of the myometrial thickness. Again in 19 (44.2%) cases the infiltration was found to be >50% (deep infiltration) and in 5 (11.6%) cases the tumour was limited to the endometrium. Extrauterine disease was observed in one of the five patients without myometrial invasion. Extrauterine disease was found in 10 of 24 (41.7%) patients who had no deep myometrial invasion: lymph node metastasis (5), adnexal metastases (5), omental implants (7), and malignant cells in abdominal cytology (6). Lymphovascular space invasion was identified in 20 (46.5%) patients.
There were 19 (44.2%) patients with Stage I, 1 (2.3%) with Stage II, 11 (25.6%) with Stage III, and 12 (27.9%) with Stage IV disease. Patient characteristics are presented in [Table/Fig-1]. According to the postoperative histopathological examinations, 25 (58.1%) patients had pure UPSC and 18 (41.8%) had mixed (serous and endometrioid) types. Overall, four mixed carcinomas were well-differentiated, 10 were moderately well-differentiated, and four were poorly differentiated.
| Parameters | Number | Percent |
|---|
| Histology | Endometrioid | 10 | 23.3 |
| Serous | 18 | 41.9 |
| Indifferent | 1 | 2.3 |
| Mixed | 9 | 20.9 |
| Unknown | 5 | 11.6 |
| Smear | Absent | 16 | 37.2 |
| Benign | 20 | 46.5 |
| AG-NOS | 1 | 2.3 |
| Adenocarcinoma | 6 | 14.0 |
| Systemic disease | Diabetes | 16 | 37.2 |
| Hypertension | 21 | 48.8 |
| Metachronous malignancy | Ovary | 4 | 9.3 |
| Colon | 1 | 2.3 |
| Breast | 3 | 7 |
| Other | 3 | 7 |
| Final histopathology | Serous | 25 | 58.1 |
| Mixed (Serous+endometrioid) | 18 | 41.8 |
| Surgical procedure | Total abdominal hysterectomy+bilaterally salphingo-oophorectomy | 43 | 100 |
| Pelvic lymph node dissection | 34 | 79.1 |
| Para-aortic lymph node dissection | 23 | 53.5 |
| Omentectomy | 28 | 65.1 |
| FIGO stage | IA | 14 | 32.6 |
| IB | 5 | 11.6 |
| II | 1 | 2.3 |
| IIIA | 6 | 14 |
| IIIB | 1 | 2.3 |
| IIIC1 | 1 | 2.3 |
| IIIC2 | 3 | 7.0 |
| IVA | 0 | 0 |
| IVB | 12 | 27.9 |
| Adjuvant treatment | None | 7 | 16.3 |
| Radiotherapy | 12 | 27.9 |
| Chemotherapy | 11 | 25.6 |
| Chemotherapy combined with radiotherapy | 13 | 30.2 |
| Site of extrauterine disease | Ovary | 13 | 30.2 |
| Pelvic lymph node | 10 | 23.2 |
| Para-aortic lymph node | 6 | 14 |
| Omentum | 12 | 27.9 |
| Carcinomatosis | 10 | 23.2 |
| Site of recurrence | Abdomen | 4 | 44.4 |
| Lymph node | 3 | 33.3 |
| Pulmonary | 1 | 11.1 |
| Multiple | 1 | 11.1 |
Adjuvant treatment was administered to 83.7% of the patients, chemo-radiotherapy to 30.2%, only radiotherapy to 27.9% and just chemotherapy to 25.6%. A total of 16.3% did not receive any adjuvant therapy. The chemotherapy regimen was carboplatin and paclitaxel every three weeks.
Of the total 43 patients, 9 (20.9%) developed disease progression and 14 (32.6%) had a relapse. Mean relapse time was 9.9 months (range: 5-17months). Two of the patients with recurrences are still alive. At the time of follow-up, 20 (46.5%) patients had survived, 21 patients had succumbed to the disease, one patient had succumbed to stroke, and one patient died due to early postoperative complications.
Median follow-up time for all patients was 20.02±17.3 months (range: 0-63.67 months), and for the survivors it was 27.36±18.77 months (range: 5.43-63.67 months). Median survival was 25.50±12.60 months (95% CI=0.799-50.201). Overall survival curve considering stages of the disease is shown in [Table/Fig-2].
Kaplan-Meier curve of overall survival by stages.
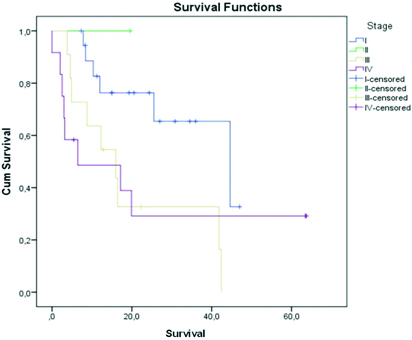
As shown in [Table/Fig-3], only advanced age and lymph node metastases were detected as statistically significant prognostic risk factors for survival.
The distribution of the prognostic variables for survival.
| Variables | Total | Mortality at follow-up | p-value |
|---|
| n | % | n | % |
|---|
| Age (≥65) years | 22 | 51.2 | 15 | 68.18 | 0.048 |
| Tumour size (≥2 mm) | 29 | 67.4 | 14 | 48.3 | 1.000 |
| Myometrial invasion (≥50%) | 19 | 44.2 | 11 | 57.9 | 0.606 |
| Lymphovascular space invasion | 20 | 46.5 | 12 | 60 | 0.425 |
| Malignant cytology | 14 | 32.5 | 9 | 64.3 | 0.330 |
| Presence of the tumour on upper abdomen | 12 | 27.9 | 8 | 66.7 | 0.281 |
| Omentectomy | 28 | 65.1 | 15 | 53.6 | 0.988 |
| Lymph node dissection | 34 | 79.1 | 17 | 50 | 0.467 |
| Lymph node involvement | 11 | 25.5 | 9 | 81.8 | 0.015 |
| Adjuvant treatment | 36 | 83.7 | 18 | 50 | 0.420 |
| Chemotherapy | 23 | 53.5 | 13 | 56.5 | 0.669 |
Pearson chi-square, Fisher’s exact test, p<0.05=significant
According to the Cox regression analysis for factors considered to have an effect on the overall survival, myometrial invasion and lymph node involvement have statistically significant effects on the overall survival (p<0.05). Results of the Cox regression analysis are represented in [Table/Fig-4]. These factors were re-evaluated by the backward stepwise Linear Regression (LR) method. Significant results are shown in [Table/Fig-5]. Cox regression analysis made by backward stepwise LR method revealed lymphovascular space invasion, lymph node involvement, and presence of the tumour in the upper abdomen to be statistically significant risk factors for the overall survival.
Cox-regression analysis for the prognostic factors considered to have an effect on overall survival.
| Variables | p | OR | 95% CI for OR |
|---|
| Lower | Upper |
|---|
| Age (≥65) years | 0.913 | 0.901 | 0.139 | 5.839 |
| Tumour size (≥2 mm) | 0.306 | 0.244 | 0.016 | 3.624 |
| Myometrial invasion (≥50%) | 0.032 | 8.659 | 1.203 | 62.315 |
| Lymphovascular space invasion | 0.155 | 0.219 | 0.027 | 1.776 |
| Malignant cytology | 0.710 | 0.533 | 0.019 | 14.736 |
| Lymph node involvement | 0.026 | 17.786 | 1.410 | 224.325 |
| Presence of the tumour on upper abdomen | 0.140 | 9.355 | 0.480 | 182.208 |
| Adjuvant treatment | 0.382 | 0.247 | 0.011 | 5.702 |
| Chemotherapy | 0.671 | 0.517 | 0.025 | 10.848 |
| Omentectomy | 0.296 | 0.251 | 0.019 | 3.355 |
Omnibus test chi-square, p<0.05=significant
The results of Cox regression analysis made by backward stepwise linear regression method for the factors considered to have an effect on overall survival.
| Variables | p | OR | 95% CI for OR |
|---|
| Lower | Upper |
|---|
| Deep myometrial invasion | 0.073 | 4.192 | 0.877 | 20.032 |
| Lymphovascular space invasion | 0.015 | 0.130 | 0.025 | 0.671 |
| Lymph node involvement | 0.005 | 7.191 | 1.808 | 28.608 |
| Presence of the tumour on upper abdomen | 0.040 | 5.181 | 1.082 | 24.805 |
Omnibus test chi-square, p<0.05=significant
Discussion
The present study was a single-institution retrospective analysis of all patients with pure and mixed UPSC managed for about a 10-year period. Mixed tumours with a serous component, even <10%, have worse prognosis than pure endometrioid adenocarcinomas and should therefore be treated as high-grade tumours [8,9]. A retrospective study by Fader AN et al., suggests that patients with a UPSC component within the tumour specimens carry a significant risk for recurrence and had lower survival rates [10]. Lawrenson K et al., suggested that molecular profiles of mixed type endometrial adenocarcinoma closely resembled the molecular profiles of pure UPSC, and even mixed cases, which are predominantly endometrioid adenocarcinomas, will have clinical courses like pure UPSC tumours [11]. Thus, in the present study pure and mixed cases of UPSC were considered together.
UPSC represents <10% of all EC [2]. In the present study it was found that UPSC constituted just 6.38% of our entire EC cohort. UPSC is generally diagnosed in more advanced age as compared to pure EC. Many authors reported mean age of UPSC subjects to be 62-70 years [12-16]. Similarly, median age of our patients was 65 years. The age over 65 years is reported to be an independent bad criterion for recurrence and poor survival in UPSC cases [17]. It was also found that advanced age (>65) years acts as a significant risk factor for poor prognosis.
UPSC frequently presents with extrauterine disease at diagnosis. Nearly half of UPSC patients present with extrauterine disease and 46% of those patients with UPSC are found to be Stage II-IV as compared to 21% for all EC. Numerous authors reported early stage/limited to the uterine corpus cases in UPSC tumours to be in the range of 31-54% [12,13,17-19]. Moreover, even in Stage I non invasive UPSC, prior studies reported up to 50% risk of occult extrauterine disease even when the disease seems to be confined to the endometrium [20]. In their retrospectively evaluated series, Ball A et al., showed that 53.1% of clinically early 64 UPSC patients (Stage I) were in fact Stage I, but 46.9% had to be upstaged after surgical staging [13].
In the present study, stage was assigned retrospectively and incompletely staged patients as well as the unevaluated sites were considered to be negative. Thus, the findings of the present study cannot determine the percentage of patients who should have been considered in the upper stages. Extrauterine disease was found in 53.5% of the present study cohort. Moreover, extrauterine disease was established in one of the five patients without myometrial invasion.
Recommendations for the surgical and adjuvant treatment for UPSC patients differ from those for pure endometrioid type endometrial carcinoma cases. Known surgical-pathologic risk factors for endometrial type endometrial carcinoma, including deep myometrial invasion, lymphovascular invasion, and tumour size, were not significantly associated with extrauterine spread in UPSC [6,10,16]. Due to higher incidence of occult extrauterine involvement, surgical staging including hysterectomy, bilateral salpingo-oophorectomy, peritoneal washings, pelvic and para-aortic lymphadenectomy and omentectomy were recommended widely, even in patients classified clinically as very early stage. Basic recommendations include surgical staging in clinically early-stage patients to determine occult extrauterine sites of the disease and designate adjuvant therapy, as well as debulking surgery in advanced stages, similarly to ovarian cancer. Unfortunately, preoperatively performed endometrial biopsies have low sensitivity rates for UPSC. Consequently, the rate of pre operatively false diagnosis as endometrioid type carcinoma is high, and 25-50% of UPSC patients are diagnosed on post operatively reported final pathology [12,21]. Therefore, great number of these cases undergo inadequate surgical treatment. In the present study cohort, 41.9% had serous tumour as the actual initial diagnosis and 20.9% had mixed endometrial carcinomas (serous and endometrioid), and 37.2% of the patients were diagnosed with UPSC on final pathology post operatively.
In the present study, surgical staging was performed including lymphatic dissection in 34 (79%) and omentectomy in 28 (65.1%) of 43 patients. In similar retrospective USPC studies, adequate surgical staging rates were reported to be 60-81% [12,13,17,18].
UPSC has a higher tendency to spread to pelvic/para-aortic lymph nodes as compared to endometrioid carcinoma. In similar retrospective UPSC studies, lymph node metastasis rates were reported to range from 21.4% to 43.8% [11,14,21,22]. Goff BA et al., reported that 42% of all patients (21/50) undergoing surgery had lymph node metastases, and of these, 10 had para-aortic nodal metastases, with or without pelvic nodal metastases. This translates into a 20% overall risk of para-aortic nodal metastases [6]. Mattes MD et al., reported that 29% of all patients had lymph node and 54.3% of them had just pelvic metastases. The 43.7% of cases with para-aortic metastases had also positive pelvic nodes [15]. However, in the present study it was found that 25.5% of cases were of lymph metastases; the rate of positive para-aortic nodes was 13.95%. Interestingly, 2.3% had metastases only in the para-aortic region. Removal of over 10 pelvic lymph nodes was considered to be adequate in a study by Mariani A et al., [23]. Mean number of extirpated lymph nodes in the present study was 29 and this was considered to be sufficient to detect occult lymphatic involvements.
Peled Y et al., investigated the effect of omentectomy on survival in a study with 52 cases. They performed omentectomy in 30 (58%) patients and found omental metastasis in 10%. A total of 77% of the cases received adjuvant chemotherapy. These authors considered that omitting omentectomy in cases with a visually normal appearing omentum has no effect on disease-free survival, time to recurrence, as well as the overall survival, and concluded that possible undetected micro-metastases in the left omentum will be influenced by adjuvant chemotherapy [21]. In the present study 12 patients, out of 28 with omentectomy, were found to have omental involvement; however, no effect of this procedure was observed on the prognosis of the disease.
The risk for recurrence of UPSC is high and increases with stage. Disease recurrence is common even in patients with non invasive serous carcinomas limited to the endometrium. Although, most patients with UPSC receive post operative adjuvant therapy, over a third of the patients (44%) develop recurrence. Most recurrences occur within the first three years of diagnosis [14,21].
UPSC tends to recur at distant sites. Huang CY et al., reported that 62.5% of patients with disease recurrence had tumours outside the pelvic cavity [12]. Similarly, Gadducci A et al., found that recurrent disease involved the peritoneum or distant sites in 66.7% of their UPSC patients [24]. Pol F et al., also found that 32/62 patients experienced a recurrence, with 81% having recurrences in their upper abdomen and/or other distant metastases [18]. In the present cohort, 14 patients (32.6%) developed relapse and all of these recurrences were in their upper abdomen and/or other distant sites. We found no solitary pelvic recurrence apart from patients with multiple recurrences.
Considering the tendency of UPSC for distant recurrence, adjuvant treatment should include both, systemic and local therapy. Chemotherapy significantly improves relapse-free survival and the overall survival in all stages [17]. Chemo-radiotherapy should be performed as adjuvant treatment for all patients with incomplete staging and also for all patients in advanced stages. Fader AN et al., investigated the role of chemotherapy in Stage I UPSC and showed that early-stage patients treated with adjuvant chemotherapy experienced significantly improved survival as compared to patients who received no adjuvant therapy or just radiotherapy [10].
Surgical-pathologic risk factors for endometrial type endometrial carcinoma, including deep myometrial invasion and tumour size, were not confirmed to be significantly associated with recurrence or survival in UPSC by several authors [6,10,16]. In the pre treatment variables, only age was prognostic for survival in the present cohort. Additionally, deep myometrial invasion and lymphovascular space invasion as surgical-pathologic risk factors affected the overall survival.
Rauh-Hain JA et al., analysed factors which influenced mortality in 13752 patients recorded in the National Cancer Database, and stated that age and advanced stage were the most important prognostic parameters. Their analysis also indicated that omitting definitive surgery as well as proper lymph node dissection is related with poor survival. They also found that chemo-radiation was associated with improved survival, but not radiotherapy or chemotherapy alone [17].
Lee LJ et al., demonstrated the importance of treatment-related factors, including optimal cytoreductive surgery as well as the use of adjuvant therapy, to decrease recurrence and improve survival in patients with advanced stage UPSC. Optimal cytoreduction is one of the most important determinants of survival in patients with advanced stage UPSC, just like epithelial ovarian cancers [16]. Similarly, Ball A et al., showed in a multivariate analysis that early stage, adjuvant chemotherapy and complete surgical staging are independent variables which prolong disease-free survival [13].
Limitation
The present study had a small sample size and comprised different stage patients. Surgical and adjuvant management of the disease may change depending on disease stage. Also, guidelines for optimal treatment changed over time. Due to relatively small sample size, it was not possible to evaluate the effectiveness of the stage and this is probably also the reason why the variables related to the therapy did not show significant effect on the prognosis. Another limitation of this study is its retrospective nature, as well as the fact that data about the cause of death of our patients are obtained from official health registrations.
Conclusion
There is an obvious need for prospective studies in UPSC patients with larger sample size and with special emphasis on lymphovascular space invasion, lymph node involvement, and presence of the tumour in the upper abdomen, as well as guidelines which may recommend more radical surgeries for the first operation.
Pearson chi-square, Fisher’s exact test, p<0.05=significant
Omnibus test chi-square, p<0.05=significant
Omnibus test chi-square, p<0.05=significant