Introduction
Testicular torsion, one of urological emergency cases, seen in neonatal or adolescent males requires early diagnosis and treatment to preserve future fertility [1]. Though the molecular mechanism of the testicular injury caused due to its torsion is yet being explored, the oxygen free radicals generated during the reperfusion period is said to cause further damage to the tissue than the ischaemic period [2]. Studies have also revealed that severity of the injury is associated to the extent and the degree of torsion, with spermatogonia and spermatocyte being the primary targets to be affected [3-5]. The association between the levels of testicular damage to different duration of ischemia has been studied in the past, in rats [6,7]. Since then, a growing number of animal studies showing the therapeutic role of various antioxidants have been documented [8,9].
Ascorbic acid, a water-soluble antioxidant, has a number of antioxidant properties and has been claimed to be the most important antioxidant in human plasma [10]. This antioxidant property of ascorbic acid is mainly related to its ability to react with many Reactive Oxygen Species (ROS) and to the fact that the resulting semidehydroascorbate is being converted back to ascorbate at the expense of Nicotinamide Adenine Dinucleotide (NADH) or reduced glutathione [11]. Ascorbic acid also contributes to the redox mechanism by salvaging other antioxidants such as vitamin E, urate and β-carotene from its oxidised form [12]. It also quenches the free radicals present in the lipid membranes, preventing lipid peroxidation when combined with the tocopherols (vitamin E) [13]. The present study was aimed to investigate the effect of ascorbic acid “an exogeneous antioxidant” on rat testicular torsion-detorsion induced injury.
Materials and Methods
Forty adult male albino rats of wistar strain, weighing 200-250 gm was divided into four groups (n=10) in the present study. Animals were housed in the animal house of Kasturba Medical College, Mangaluru, Karnataka, India. This study was conducted as a part of PhD thesis from 2004-2007. The duration of the experimental work was about 16 months. Seven days of acclimatisation period was given to the animals before the commencement of experiment.
The protocol carried out in this animal experiment was reviewed and approved by the Local Committee on Medical Ethics for the use of laboratory animals of Kasturba Medical College, Karnataka, India.
The animals of Group I served as normal control while Group II animals underwent Sham operation. Group III animals were subjected to manual rotation of the testes (720° clockwise) for three hours followed by counter rotation (detorsion) for one hour without any treatment. Group IV animals were pretreated with ascorbic acid dissolved in distilled water (40 mg/kg.bw, orally) for a period of 30 days before undergoing three hours testicular torsion followed by one-hour detorsion. At each time point, animals were anaesthetised by pentobarbitone sodium (40 mg/kg.bw intraperitoneal) under strict aseptic conditions. Scrotectomy, was performed through mid scrotal vertical incision for inducing torsion and detorsion. The left testis was manually rotated 720° clockwise to induce ischaemia. A silk suture was thereafter passed through its tunica albuginea to fix the testis to the scrotum to retain the same position. Counter rotation of the testis to its original alignment for one hour was performed to allow reperfusion, which was confirmed by observing the development of reactive hyperaemia. Animals that failed to develop reactive hyperaemia were excluded from the study. The testis was covered with gauze soaked in normal saline (0.9% sodium chloride) to keep it moist. After each surgical procedure, the incision was closed and the animals were allowed to recover from anaesthesia.
They were left free in their cages and were fed with standard laboratory pellet diet (Gold Mohur, Lipton India Ltd.,) and water ad libitum. The animals were sacrificed at the end of one hour of detorsion using a lethal dose of sodium pentobarbitone (100 mg/kg.bw). Sequential biopsies were immediately performed where testes were removed for morphological and biochemical evaluation.
Histopathological analysis of the testis: The testicular tissue removed for histopathological evaluation was processed and paraffin blocks were prepared as per standard protocols [14]. Sections of 5μ thickness were obtained, stained with haematoxylin and eosin for light microscopic analysis.
A-Quantitative Analysis of Testicular Damage
Measurement of Standard Tubular Diameter (STD): Five transversely cut sections from various fields of each testis was evaluated using a stage micrometer that was calibrated with ocular micrometer. Short and long diameter of the tubule, one perpendicular to the other was measured and the average of these two diameters was taken for each animal [15].
Measurement of Standard Epithelial Height (SEH): From each transversely cut sections of the testis, five seminiferous tubules per slide were randomly picked and measured for their epithelial height [16].
B-Qualitative Evaluation
Testicular tissue damage was graded conferring the presence of coagulation type necrosis detected in the sections of seminiferous tubules [17].
Grade 0: Absence of coagulation and necrosis.
Grade 1: Mild coagulation, with less than 25% of the tubules exhibiting necrosis varying from lack of spermatogenesis, disruption in the germinal layers and necrosis in individual cells.
Grade 2: Moderate coagulation, with 25% to 75% of the tubules presenting variable grades of necrosis ranging from lack of spermatogenesis with disruption and damage of maturation layers to necrosis in individual cells, considering common features of Grade 1 and Grade 3 evaluation.
Grade 3: Severe coagulation, with 75% or more of the tubules showing total necrosis.
Biochemical Analysis
Estimation of testicular lipid peroxidation: This assay was performed based upon the reaction of Thiobarbituric Acid (TBA) with Malondialdehyde (MDA) that is one of the aldehyde products of lipid peroxidation [18]. Spectronic D-20 spectrophotometer was used to measure the formation of a pink colour at 535 nm. TBA was expressed in terms of nanomoles of MDA/g of wet tissue.
Estimation of tissue Glutathione (GSH): Tissue GSH form the homogenate {10% w/v in 10 mM Phosphate-Buffered Saline (PBS; pH. 7.4)} was estimated [19]. Optical density was measured at 412 nm using a Spectronic D-20 spectrometer and was expressed as GSH in nmol/mg of tissue protein.
Superoxide Dismutase (SOD) assay: SOD was estimated as per the method of Kakkar P et al., [20]. The reduction of Nitro Blue Tetrazolium (NBT) by O2-was measured at 560 nm using a Spectron D-20 spectrophotometer and the values were expressed in unit/mg protein of tissue homogenate.
Chemicals: Riboflavin, TBA, NBT, Reduced GSH, and L-Methionine were procured from Loba Chem Pvt. Ltd., Mumbai, India. Trichloroacetic acid and Folins reagent were obtained from Qualigens Fine Chemicals, India. Ethylenediaminetetraacetic acid was purchased from SDFCL Ltd., Mumbai, India. Dithiobis-2 Nitro benzoic acid and Ascorbic acid were bought from SISCO Research Laboratories Ltd., Mumbai, India. All chemicals used in this study were of analytical grade.
Statistical Analysis
All values in the text and figures are presented as Mean±Standard Deviation (SD). Statistical analysis was calculated using SPSS package version II. The analysis of multiple group variation was done by ANOVA. The post-hoc (LSD) test was done for inter-group comparison. Statistical significance was considered as p<0.05.
Results
Biochemical Assay
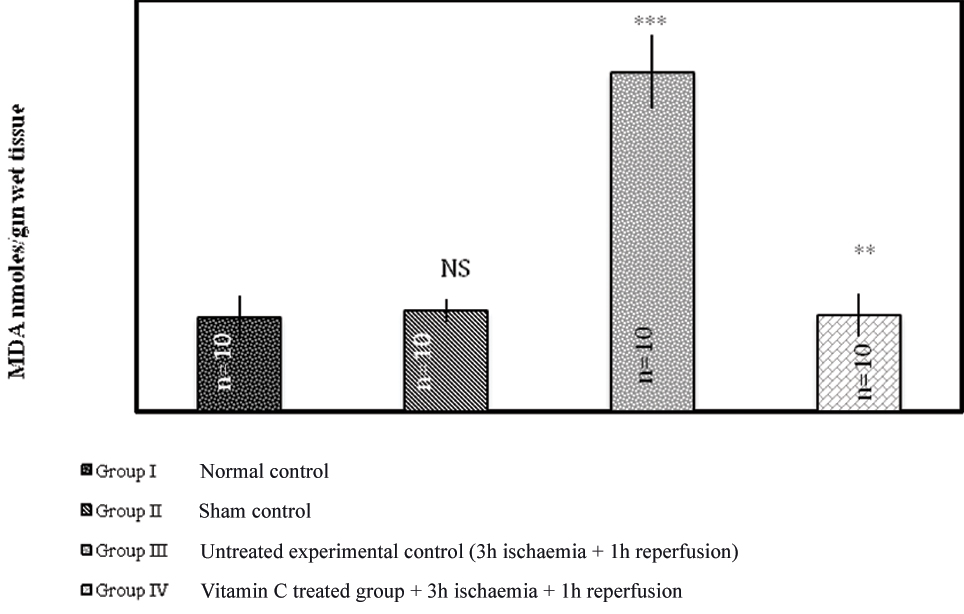
Tissue lipid peroxidation (MDA assay): A significant increase in the level of tissue lipid peroxidation was observed in the untreated experimental group animals (Group III) and the animals pre-treated with ascorbic acid (Group IV) on the other hand showed a significant reduction in the level of lipid peroxidation compared to the untreated experimental control group [Table/Fig-1].
Effect of 3 hour of ischaemia followed by reperfusion for 1 hour on the levels of tissue MDA.
The values are expressed as mean±SD, n=number of animals used in the study
*** p<0.0001 versus Gr II and Gr I. ** p<0.0001 versus Gr III. NS=Not significant versus Gr I.
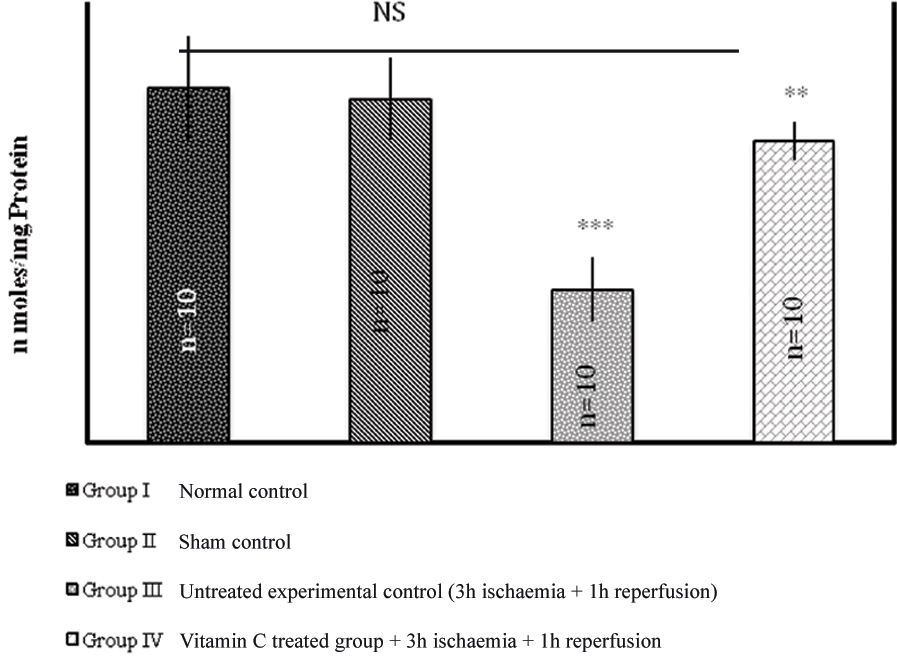
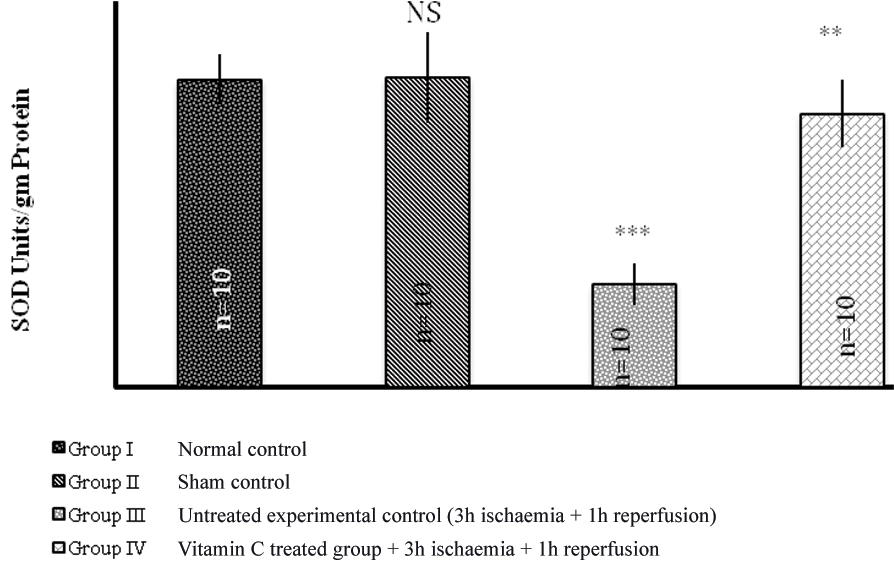
Tissue glutathione (GSH) and tissue superoxide dismutase (SOD): A significant decrease in the level of tissue GSH and SOD was observed in the animals of Group III when compared to normal control and sham control after 3 hour of torsion and 1hour of reperfusion (p<0.0001) as depicted in [Table/Fig-2-3]. However, the animals that were pre-treated with ascorbic acid (Group IV) showed near normal values to that of their control groups.
Effect of 3 hour of ischaemia followed by reperfusion for 1 hour on the levels of tissue GSH.
The values are expressed as mean±SD, n=number of animals used in the study.
*** p<0.0001 versus Gr II and Gr I. ** p<0.0001 versus Gr III. NS=Not significant versus Gr I.
Effect of 3 hour of ischaemia followed by 1 hour of reperfusion on the levels of tissue SOD.
The values are expressed as mean±SD, n=number of animals used in the study.
*** p<0.0001 versus Gr II and Gr I. ** p<0.0001 versus Gr III and Gr I. NS=Not significant versus Gr I.
Histopathological Evaluation
Quantitative analysis of testicular damage: The changes in STD and SEH, of the testis following 3 hour of ischaemia followed by reperfusion for 1 hour are summarised in [Table/Fig-4]. The animals in Group III showed a significant reduction in the STD and SEH compared to that of control groups (Group I and Group II). However, the animals of the group that was pre-treated with ascorbic acid (Group IV) prior to induction of 3 hour ischaemia followed by 1 hour reperfusion showed a near normal value in their STD and SEH [Table/Fig-4].
Effect of 3 hour of ischaemia followed by reperfusion for 1 hour on STD and SHE.
| Groups | STD (μm) | SEH (μm) |
|---|
| I (Normal control) | 597.51±28.4 (n=10) | 75.14±9.38 (n=10) |
| II (Sham control) | 561.76±38.5 NS (n=8) | 76.35±4.60 NS (n=8) |
| III (Untreated experimental control) | 450.42±66.2*** (n=8) | 34.54±7.83*** (n=8) |
| IV (Pre-treated with ascorbic acid) | 595.09±64.72** (n=8) | 63.63±7.68** (n=8) |
The values are expressed as mean±SD; n=sample size.
STD: ***=p<0.0001 versus Gr. I and Gr. II, **=p<0.0001 versus Gr. III. NS=Not significant versus Gr. I.
SEH: ***=p<0.0001 versus Gr. I and Gr. II,**=p<0.0001 versus Gr. III. NS=Not significant versus Gr. I.
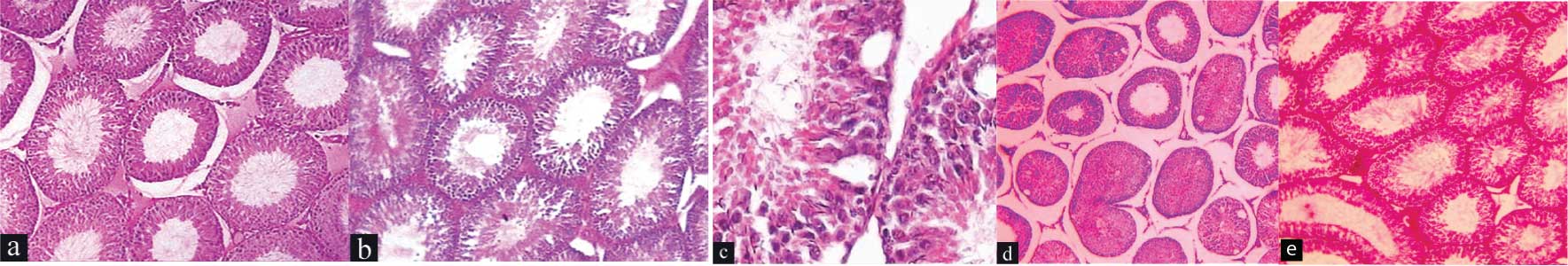
Qualitative evaluation: Animals of Group I and Group II showed normal testicular architecture with an orderly arrangement of germinal cells with absence of coagulation and necrosis in the STD [Table/Fig-5] respectively. In Group III, coagulative necrosis of germinal cells was observed within the STD with 75% or more of the tubules showing complete necrosis (Grade 3). On the other hand, the animals of the Group IV showed disordered, sloughed germinal cells within the STD and 25% or more of the tubules showed variable degrees of necrosis (Grade 2).
Photomicrograph of rat testis in different groups: a) Normal control group (group I) showing normal histological features (H&E, 10X); b) Sham control (group II) showing normal histological features in (H&E, 10X); c) Experimental control group rats that underwent 3 hours of testicular torsion and 1-hour counter-rotation (group III) showing cytoplasmic vacuoles in the seminiferous tubules of (H&E, 40X); d) Experimental control group rats that underwent 3 hours of testicular torsion and 1-hour counter-rotation (group III) showing necrosis in more than 75 percent of the seminiferous tubules (H&E, 10X); e) Pretreated experimental group of rats that were pre-treated with ascorbic acid prior to the induction of 3 hours of testicular torsion followed by counter-rotation for 1 hour (group IV) showing 25 percent or more necrosis of variable degrees in the seminiferous tubules (H&E, 10X).
Discussion
In the present study, the tissue levels of MDA were significantly increased in the testes of the untreated experimental control group rats (p<0.0001) when compared to that of normal and sham control group confirming oxidative injury that occurred in the testicular tissue. It has been documented that reduction in blood flow leads to hypoxia of the organs resulting in high levels of tissue lipid peroxide products [21]. Reperfusion of the ischaemic tissue will further lead to the formation of ROS and can additionally cause damage and atrophy of the testicular tissue [22]. MDA, a final product of lipidperoxidation, is used in assessing the formation of ROS after restoring the blood supply to the tissues [23,24]. This is agreeable in the present study as the animals that underwent testicular torsion and detorsion showed a fourfold increase in the levels of lipid peroxidation [Table/Fig-1] which simply proves that testes promotes severe cell membrane peroxidation [24]. Usually in the initial phases the endogeneous antioxidants are said to counterbalance the injury. However, upon production of ROS beyond the capacity of defense mechanism the tissue injury occurs [25].
When the animals were pretreated with ascorbic acid prior to torsion and detorsion, a significant reduction in the level of tissue MDA indicates the prevention of lipid peroxidation. Reports show that rise in levels of oxidants or reduction in the tissue antioxidant levels is proved to be an indicator of oxidative stress [26]. Reduced concentration of GSH and SOD in the animals of untreated group in the present study ratifies the above statement [Table/Fig-2,3].
The duration of torsion chosen in the present study was based on the reports of Abasiyanik A and Dagdonderen L [27]. According to them one hour of torsion lead to loss of germ cells while two hours and above lead to loss of sertoli cells in adult rats. The histopathological evaluation in the present study showed decreased STD and SEH in the testis of animals of the untreated experimental group (Group III; [Table/Fig-4]), agreeing with the suggestion of above-mentioned authors. Besides, the histological sections of the seminiferous tubules in this group also showed interstitial oedema, vacuolisation, maturation arrest, haemorrhage and coagulative necrosis of germinal cells with 75% or more of the seminiferous tubules showing complete necrosis. Numerous intercellular areas were also observed inbetween the germinal cells lining the seminiferous tubules [Table/Fig-5] which is said to be caused due to the loosening of inter cellular connections that finally leads to shrinkage of both germ and sertoli cells [28].
On the other hand, there was no significant reduction/damage in the STD and SHE of the tubules in the animals of the pretreated group (Group IV; [Table/Fig-4]). This suggests that pretreatment with ascorbic acid probably helped in maintaininOn the other hand, there was no significant reduction/damage in the STD and SHE of the tubules in the animals of the pretreated group (Group IV; [Table/Fig-4]). This suggests that pretreatment with ascorbic acid probably helped in maintaining the normal architecture of the testisg the normal architecture of the testis and the seminiferous tubules without affecting the spermatogenesis.
Various researches have examined the effects of moderate and sustained reduction in testicular blood and its effects on the testis [7,29]. They observed that reductions in blood flow might play a major role in pathogenesis of male infertility. Aktas BK et al., demonstrated that administration of N-acetylcysteine to animals, prior to torsion and detorsion significantly increased the STD and SHE, in their experimental study [30]. Similar results of significantly less tissue injury to the seminiferous tubules were witnessed by Kemahli E et al., and his coworkers when the animals were pretreated with pyrrolidine dithiocarbamate15 minutes prior to detorsion [31]. In the present study, those animals that were pretreated with ascorbic acid and then underwent torsion-detorsion showed significantly less tissue damage. Testicular torsion and detorsion in animals exemplifies a practical model to that of humans and the production of ROS is the main cause of tissue damage. Growing evidence show that testicular torsion related injuries in newborns and young adolescents are being reversed when diagnosed and treated early [32]. Since exogenous antioxidants like vitamin C and E have proven beneficial in treating male infertility [33], the administration of ascorbic acid while restoring the blood flow after torsion in newborns and young adolescents, may potentially play a vital role in scavenging the free radicals produced during the tissue injury.
Limitation
A long-term follow-up particularly after the postoperative procedures to further check the testicular viability in the animals could not be performed. Since the contralateral testis was not investigated, a parallel investigation of both testes might provide further insight to this study. Moreover, we could not afford the cost to include any ROS mediated immunohistochemistry stains as this project was not funded.
Conclusion
Present study result deliberates that pretreatment with ascorbic acid significantly reduced testicular tissue damage induced by elevated level of ROS during testicular torsion-detorsion. Therefore, administration of ascorbic acid in the patients with testicular torsion from the time of diagnosis until the surgery and during recovery period might minimise the damage and salvage the testicular function.
[1]. Ta A, D’Arcy FT, Hoag N, D’Arcy JP, Lawrentschuk N, Testicular torsion and the acute scrotum: current emergency managementEur J Emerg Med 2016 23:160-65.10.1097/MEJ.000000000000030326267075 [Google Scholar] [CrossRef] [PubMed]
[2]. Al-Maghrebi M, Renno WM, The tACE/angiotensin (1–7)/mas axis protects against testicular ischemia reperfusion injuryUrology 2016 94:e1-8.10.1016/j.urology.2016.04.02127125877 [Google Scholar] [CrossRef] [PubMed]
[3]. Dursun R, Zengin Y, Gündüz E, İçer M, Durgun HM, Dağgulli M, The protective effect of goji berry extract in ischemic reperfusion in testis torsionInt J Clin Exp Med 2015 8(2):2727 [Google Scholar]
[4]. Parlaktas BS, Atilgan D, Ozyurt H, Gencten Y, Akbas A, Erdemir F, The biochemical effects of ischemia-reperfusion injury in the ipsilateral and contralateral testes of rats and the protective role of melatoninAsian J Androl. 2014 16(2):31410.4103/1008-682X.12220224407181 [Google Scholar] [CrossRef] [PubMed]
[5]. Yang C, Song B, Tan J, Liu X, Wei GH, Testicular torsion in children: a 20-year retrospective study in a single institutionSci World J 2011 11:362-68.10.1100/tsw.2011.3921336452 [Google Scholar] [CrossRef] [PubMed]
[6]. Sukhotnik I, Miselevich I, Lurie M, Nativ O, Coran AG, Mogilner JG, The time relationship between ipsilateral testicular ischemia and germ cell apoptosis in the contralateral testis in ratPediatr Surg Int 2005 21(7):512-16.10.1007/s00383-005-1477-716025273 [Google Scholar] [CrossRef] [PubMed]
[7]. Kurt O, Yazici CM, Gevher F, Balci H, Yitik A, Ozkara H, The effect of testicular torsion duration on testicular steroidogenesis in the rat modelUrol Int 2016 97(3):358-64.10.1159/00044396927115502 [Google Scholar] [CrossRef] [PubMed]
[8]. Hekimoglu A, Kurcer Z, Aral F, Baba F, Sahna E, Atessahin A, Lycopene, an antioxidant carotenoid, attenuates testicular injury caused by ischemia/reperfusion in ratsTohoku J Exp Med 2009 218(2):141-47.10.1620/tjem.218.14119478470 [Google Scholar] [CrossRef] [PubMed]
[9]. Ribeiro CT, Milhomem R, De Souza DB, Ribeiro WS, Sampaio FJ, Pereira-Sampaio MA, Effect of antioxidants on outcome of testicular torsion in rats of different agesJ Urol 2014 191(5):1578-85.10.1016/j.juro.2013.09.06624679870 [Google Scholar] [CrossRef] [PubMed]
[10]. Angulo C, Maldonado R, Pulgar E, Mancilla H, Córdova A, Villarroel F, Vitamin C and oxidative stress in the seminiferous epitheliumBiological Research 2011 44(2):169-80.10.4067/S0716-9760201100020000922513420 [Google Scholar] [CrossRef] [PubMed]
[11]. Frei B, England L, Ames BN, Ascorbate is an outstanding antioxidant in human blood plasmaProceedings of the National Academy of Sciences 1989 86(16):6377-81.10.1073/pnas.86.16.63772762330 [Google Scholar] [CrossRef] [PubMed]
[12]. Halliwell B, Gutteridge JM, Oxygen free radicals and iron in relation to biology and medicine: some problems and conceptsArch Biochem Biophys 1986 246:501-14.10.1016/0003-9861(86)90305-X [Google Scholar] [CrossRef]
[13]. Neužil J, Thomas SR, Stocker R, Requirement for, promotion, or inhibition by α-tocopherol of radical-induced initiation of plasma lipoprotein lipid peroxidation.Free Radic Biol Med 1997 22(1):57-71.10.1016/S0891-5849(96)00224-9 [Google Scholar] [CrossRef]
[14]. Culling CFA, Allison RT, Barr WT, Cellular pathology technique 1985 884th EditionLondonButterworths:5510.1016/B978-0-407-72903-2.50031-9 [Google Scholar] [CrossRef]
[15]. Canan S, Šahin B, Odaci E, Ünal B, Aslan H, Bilgic S, Estimation of the reference volume, volume density and volume ratios by a stereological method: Cavalieri’s principleTurkiye Klinikleri J Surg Med Sci 2002 22(1):7-48. [Google Scholar]
[16]. Koruji M, Movahedin M, Mowla SJ, Gourabi H, Jabbary Arfaee A, The morphological changes of adult mouse testes after 60Co γ-radiationIran Biomed J 2008 12(1):35-42. [Google Scholar]
[17]. Greenstein A, Smith-Harrison LI, Wakely PE, Kololgi S, Salzberg AD, Koontz WW, The effect of polyethylene glycol-superoxide dismutase administration on histological damage following spermatic cord torsionJournal urology 1992 148(2):639-41.10.1016/S0022-5347(17)36678-8 [Google Scholar] [CrossRef]
[18]. Jiang ZY, Hunt JV, Wolff SP, Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoproteinAnal Biochem 1992 202(2):384-89.10.1016/0003-2697(92)90122-N [Google Scholar] [CrossRef]
[19]. Laurence RA, Burk RF, Glutathion peroxidase activity in selenium-dificient rat liver.Biochem Biophys Res Comm 1976 71:952-58.10.1016/0006-291X(76)90747-6 [Google Scholar] [CrossRef]
[20]. Kakkar P, Das B, Viswanathan PN, A modified spectrophotometric assay of superoxide dismutaseIndian J Biochem Biophys 1984 21(2):130-32. [Google Scholar]
[21]. Lee SM, Park MJ, Cho TS, Clemens MG, Hepatic injury and lipid peroxidation during ischemia and reperfusionShock 2000 13(4):279-84.10.1097/00024382-200004000-0000510774616 [Google Scholar] [CrossRef] [PubMed]
[22]. Kim YH, Kim GH, Shin JH, Kim KS, Lim JS, Effect of korean red ginseng on testicular tissue injury after torsion and detorsionKorean J Urol 2010 51(11):794-99.10.4111/kju.2010.51.11.79421165202 [Google Scholar] [CrossRef] [PubMed]
[23]. Takhtfooladi MA, Jahanshahi A, Sotoudeh A, Khansari M, Takhtfooladi HA, The antioxidant role of N-acetylcysteine on the testicular remote injury after skeletal muscle ischemia and reperfusion in ratsPol J Pathol 2013 64(3):204-09.10.5114/pjp.2013.3814024166607 [Google Scholar] [CrossRef] [PubMed]
[24]. Guimarães SB, Aragão AA, Santos JM, Kimura OD, Barbosa PH, Vasconcelos PR, Oxidative stress induced by torsion of the spermatic cord in young ratsActa Bras Cir 2007 22(1):30-33.10.1590/S0102-8650200700010000517293946 [Google Scholar] [CrossRef] [PubMed]
[25]. Lysiak JJ, Nguyen QA, Turner TT, Peptide and nonpeptide reactive oxygen scavengers provide partial rescue of the testis after torsionJ Androl 2002 23(3):400-09. [Google Scholar]
[26]. Durackova Z, Knasmuller S, The Activity of Natural Compounds in diseases prevention and therapySAP, Bratislava, Slovakia 2007 :11-59. [Google Scholar]
[27]. Abasiyanik A, Dağdğnderen L, Beneficial effects of melatonin compared with allopurinol in experimental testicular torsionJ Pediatr Surg 2004 39(8):1238-41.10.1016/j.jpedsurg.2004.04.01815300535 [Google Scholar] [CrossRef] [PubMed]
[28]. de Souza PF, Monteiro JC, Matta SL, Garcia MC, Testicular histomorphometry and ultrastructure of rats treated with cadmium and Ginkgo bilobaBiol Trace Elem Res 2011 140(3):330-41.10.1007/s12011-010-8702-520428964 [Google Scholar] [CrossRef] [PubMed]
[29]. Ramachandra P, Palazzi KL, Holmes NM, Marietti S, Factors influencing rate of testicular salvage in acute testicular torsion at a tertiary pediatric centerWest J Emerg Med. 2015 16(1):190-94.10.5811/westjem.2014.11.2249525671040 [Google Scholar] [CrossRef] [PubMed]
[30]. Aktaş BK, Bulut S, Bulut S, Baykam MM, Özden C, Šenes M, The effects of N-acetylcysteine on testicular damage in experimental testicular ischemia/ reperfusion injuryPediatr Surg Int 2010 26(3):293-98.10.1007/s00383-009-2538-019911182 [Google Scholar] [CrossRef] [PubMed]
[31]. Kemahli E, Yildiz M, Firat T, Özyalvaçli ME, Üyetürk U, Yilmaz B, An experimental study on effects of pyrrolidine dithiocarbamate on ischemiareperfusion injury in testisCanadian Urolo Assoc J 2016 10(3-4):E10410.5489/cuaj.316027330576 [Google Scholar] [CrossRef] [PubMed]
[32]. Drlík M, Kočvara R, Torsion of spermatic cord in children: a reviewJ Pediatr Urol 2013 9(3):259-66.10.1016/j.jpurol.2012.05.01622763105 [Google Scholar] [CrossRef] [PubMed]
[33]. Sheweita SA, Tilmisany AM, Al-Sawaf H, Mechanisms of male infertility: role of antioxidantsCurr Drug Metab 2005 6(5):495-501.10.2174/13892000577433059416248841 [Google Scholar] [CrossRef] [PubMed]