Pseudomonas monteilii an Emerging Pathogen in Meningoencephalitis
Varsha Gupta1, Shiwani Sharma2, Lipika Singhal3, Ranu Soni4, Jagdish Chander5
1 Professor, Department of Microbiology, Government Medical College and Hospital, Sector 32, Chandigarh, India.
2 Demonstrator, Department of Microbiology, Government Medical College and Hospital, Sector 32, Chandigarh, India.
3 Assistant Professor, Department of Microbiology, Government Medical College and Hospital, Sector 32, Chandigarh, India.
4 Junior Resident, Department of Microbiology, Government Medical College and Hospital, Sector 32, Chandigarh, India.
5 Professor and Head, Department of Microbiology, Government Medical College and Hospital, Sector 32, Chandigarh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shiwani Sharma, Demonstrator, Department of Microbiology, Level 1, E Block, Government Medical College and Hospital, Sector 32, Chandigarh-160030, India.
E-mail: drshiwani@gmail.com
Pseudomonas monteilii (P. monteilii) is a gram negative, rod shaped, nonsporing, motile, non-fermenting bacterium belonging to family Pseudomonadaceae. It is a known environmental contaminant, has been seen as an emerging opportunistic pathogen and is closely related to Pseudomonas putida. We isolated this organism from a young immunocompetent patient with meningoencephalitis, who despite of treatment had a fatal outcome.
Mass spectrometry, Non-aeruginosa pseudomonas, Nosocomial
Case Report
A 21-year-old male presented to emergency medicine department with chief complaints of fever which was recorded as 101°F for the past seven days, headache and four episodes of vomiting for the last one day. On taking the detailed history, it was revealed that the fever was associated with chills and rigor along with headache which was primarily in the frontal region. The patient had generalised body weakness, retro-orbital pain with blurring of vision for the same duration. No history suggestive of any abnormal behaviour, altered consciousness, loose stools with or without blood etc., was elicited.
On examination temperature was 100°F, Blood Pressure (BP) 130/86 mm in supine position and pulse rate was 100 beats/minute. General physical examination was within normal limits. On Central Nervous System (CNS) examination patient was conscious, oriented to time, place and person and there was no neck rigidity at the time of presentation.
Routine blood examination was done and blood tests revealed an elevated leukocyte count (27,400/μL), elevated Erythrocyte Sedimentation Rate (ESR) (42 mm/hour) and elevated C-reactive protein level (25 mg/dL). Cerebrospinal Fluid (CSF) examination revealed Total Leukocyte Count (TLC) 48/mm3, Differential Leukocyte Count (DLC) showed 60% neutrophils, 40% lymphocytes. The CSF was clear, and the concentrations of glucose and total protein in the CSF were 31 mg/dL and 65 mg/dL, respectively. Direct wet mount of CSF showed 4-5 WBC per low power field and no RBCs per low power field were seen. Direct Gram staining revealed no bacteria with few pus cells. The CSF was sent for bacterial culture and sensitivity. The patient was put on ceftriaxone 2g IV 12 hourly and vancomycin 15 mg/kg IV 8 hourly for seven days and shifted to medicine ward; this was done as part of antibiotic protocol for management which is followed by Department of Medicine at present hospital giving coverage to both Gram positive and Gram negative bacteria. However, patient did not respond with these antibiotics. Non-Contrast Computerised Tomography (NCCT) head revealed normal findings. The HIV and Hepatitis B serology showed non-reactive results.
In the Microbiology laboratory, CSF sample was inoculated on 5% sheep blood agar, chocolate agar and MacConkey agar and incubated at 37°C for 24 hours. The pure growth of non-haemolytic colonies with metallic sheen on blood agar, large mucoid colonies on chocolate agar and non-lactose fermenting colonies on MacConkey agar, were obtained after 24 hours [Table/Fig-1,2]. Gram stain from all these media showed gram negative, rod shaped bacteria with no specific arrangement. The isolate was catalase and oxidase positive. Biochemical reactions results are as follows: On Triple Sugar Iron (TSI) agar the organism had a reaction of alkaline/alkaline with no change in growth with no hydrogen sulphide production and no gas production, Oxidative/Fermentative (OF) reaction was oxidative. It was Simmon’s citrate positive, indole test negative, urease negative and methyl red reaction was negative. L-arginine dihydrolase positive and gave negative reactions for L-lysine decarboxylase and ornithine decarboxylase. This motile organism with slightly reddish brown pigment was conventionally identified as Pseudomonas species based on these standard biochemical tests [Table/Fig-3] [1]. Further, for confirmation the organism was sent for Matrix-Assisted Laser Desorption/Ionisation-Time of Flight Mass Spectrometry (MALDI-TOF-MS). The isolate was confirmed as P. monteilii. Antibiotic Susceptibility Testing (AST) was performed on Mueller Hinton agar by Kirby Bauer disc diffusion method [2]. The isolate was found to be sensitive to polymyxin B, colistin and tetracycline and resistant to amikacin, imipenem, aztreonam, ceftazidime, ciprofloxacin, piperacillin and tazobactam as per Clinical and Laboratory Standard Institute (CLSI) Guidelines [3]. Blood and urine cultures of the patient were sterile. Repeat CSF biochemistry revealed marked raised proteins i.e. 313 mg/dL and glucose 37 mg/dL and same culture results. Patient was put on parenteral colistin (2 million units IV BD) and showed some signs of recovery. He left from hospital against medical advice and was declared as Leave Against Medical Advice (LAMA). On telephonic follow up, the relatives revealed that he died after coming home and died after two days of his discharge.
Large mucoid colonies on chocolate agar.
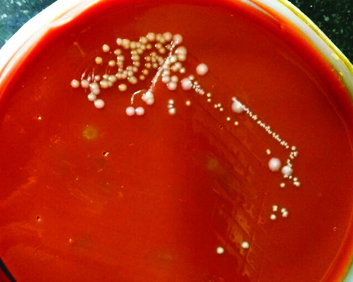
Non-lactose fermenting colonies on MacConkey agar.

Reddish brown pigment of isolate on nutrient agar slant.

Discussion
Pseudomonas spp. are important opportunistic and nosocomial pathogens. The common human pathogens in this genus include Pseudomonas aeruginosa and other non-aeruginosa Pseudomonas like Pseudomonas putida, Pseudomonas stutzeri etc. Pseudomonas aeruginosa has become one of the most serious causes of nosocomial bacterial infection notably in the lung, blood and urinary tract. Other non-aeruginosa Pseudomonas though less virulent, are also opportunistic pathogens and may cause colonisation of the airways and other sites. A study by Huang CR et al., showed 11 cases of Adult Bacterial Meningitis (ABM) caused by non-aeruginosa Pseudomonas spp. [4]. P. monteilii is a species closely related to P. putida based on 16S rRNA analysis so it has been placed in the P. putida group [5]. It is a versatile bacterium that can be isolated from different natural environments [6,7]. P. monteilii is an uncommon but increasingly recognised organism in the hospital environment and has been cultured most frequently from soil, garbage and drains [8]. Most isolates are environmental, including resistant strains from the hospital environment [9]. So, accurate identification of less common Pseudomonas spp. like P. monteilii is important.
In the present case report we isolated P. monteilii from 21-year-old male with meningoencephalitis from CSF sample with clinically no sign of neck rigidity. A study by Thomas KE et al., also demonstrated that these diagnostic tools (clinical signs like neck rigidity) are too insensitive to identify the majority of patients with meningitis in contemporary practice [10]. The present species is probably under reported due to difficulty in identification. Though, it is considered a coloniser and potential pathogen, its status as a human pathogen is unclear and remains unreported despite of its significant pathogenicity. Though, infections with Pseudomonas are usually attributed to acquisition of organism in hospitals particularly in patients on mechanical ventilation, antibiotic treatment, chemotherapy or surgery. However, in present case no such history of any trauma or surgery had been elicited [11]. So, the exact source cannot be mentioned. Probably it is from the environment only. In an earlier study it has been shown that P. monteilii was responsible for causing exacerbation of bronchiectasis and was isolated from sputum sample [12].
Conclusion
P. monteilii is a relatively rare non-aeruginosa species and relatively resistant organism as also seen in present strain of P. monteilii which is associated with severe fatal infections. Thus, early diagnosis and treatment should be advocated. Further, the purpose of the discussion and presentation of this organism is important to make the microbiologists and clinicians aware of the new pathogen.
[1]. Collee JG, Duguid JP, Fraser AG, Marmion BP, Simmons A, A. Laboratory strategy in the diagnosis of infective syndromes. In: Collee JG, Fraser AG, Marmion BP, Simmons A (eds) Mackie and MacCartney practical medical microbiology 2014 14th EditionLondonChurchill Livingstone:53-94. [Google Scholar]
[2]. Bauer AW, Kirby WMM, Sherris JC, Turck M, Antibiotic susceptibility testing by standardised single disk methodAm J Clin Pathol 1966 45(1):93-96.10.1093/ajcp/45.4_ts.4935325707 [Google Scholar] [CrossRef] [PubMed]
[3]. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute 2017 27th Edition [Google Scholar]
[4]. Huang CR, Lien CY, Tsai WC, Lai WA, Hsu CW, Tsai NW, The clinical characteristics of adult bacterial meningitis caused by non-Pseudomonas (Ps.) aeruginosa Pseudomonas species: a clinical comparison with Ps. aeruginosa meningitisKaohsiung J Med Sci 2018 34(1):49-55.10.1016/j.kjms.2017.08.00729310816 [Google Scholar] [CrossRef] [PubMed]
[5]. Anzai Y, Kim H, Park JY, Wakabayashi H, Oyaizu H, Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequenceInt J Syst Evol Microbiol 2000 50:1563-89.10.1099/00207713-50-4-156310939664 [Google Scholar] [CrossRef] [PubMed]
[6]. Scotta C, Juan C, Cabot G, Oliver A, Lalucat J, Bennasar A, Environmental microbiota represents a natural reservoir for dissemination of clinically relevant metallo-β-lactamasesAntimicrob Agents Chemother 2011 55(11):5376-79.10.1128/AAC.00716-1121859934 [Google Scholar] [CrossRef] [PubMed]
[7]. Ma Q, Qu Y, Tang H, Yu H, Ma F, Shi S, Genome sequence of a novel indigoproducing strain, Pseudomonas monteilii QMJ Bacteriol 2012 194(16):4459-60.10.1128/JB.00867-1222843591 [Google Scholar] [CrossRef] [PubMed]
[8]. Remold SK, Brown CK, Farris JE, Hundley TC, Perpich JA, Purdy ME, Differential habitat use and niche partitioning by Pseudomonas species in human homesMicrob Ecol 2011 62(3):505-17.10.1007/s00248-011-9844-521503776 [Google Scholar] [CrossRef] [PubMed]
[9]. Bogaerts P, Bouchahrouf W, Lissoir B, Denis O, Glupczynski Y, IMP-13-producing Pseudomonas monteilii recovered in a hospital environmentJ Antimicrob Chemother 2011 66(10):2434-35.10.1093/jac/dkr29421764825 [Google Scholar] [CrossRef] [PubMed]
[10]. Thomas KE, Hasbun R, Jekel J, Quagliarello VJ, The diagnostic accuracy of kernig’s sign, brudzinski’s sign, and nuchal rigidity in adults with suspected meningitisClinical Infectious Diseases 2002 35(1):46-52.10.1086/34097912060874 [Google Scholar] [CrossRef] [PubMed]
[11]. Pier GB, Ramphal R, Pseudomonas aeruginosa. In:Mandell GL, Bennett JE, Dolin R(eds). Principles and practice of infectious diseases 2005 6th EditionChurchillLivingstone:2587-2615. [Google Scholar]
[12]. Aditi A, Shariff M, K Beri, Exacerbation of bronchiectasis by Pseudomonas monteilii:a case reportBMC Infect Dis 2017 17(1):51110.1186/s12879-017-2600-928738817 [Google Scholar] [CrossRef] [PubMed]