Introduction
Any kind of glaucoma that does not respond to medical or conventional surgical treatment is called refractory glaucoma [1]. This type of glaucoma is a significant clinical problem because of frequency of occurrence, difficulty in diagnosis and complex management [2]. The term refractory glaucoma encompasses a wide variety of presentations and mechanisms like post-vitrectomy glaucoma, post-penetrating keratoplasty glaucoma, neovascular glaucoma, uveitic glaucoma, paediatric glaucoma, failed filtration surgeries and post-traumatic glaucoma [3]. The management of these intractable patient’s essentially remains surgical re-intervention and poses a huge challenge to an ophthalmologist.
Various modifications in trabeculectomy such as use of anti-fibrotic agents and mechanical barriers have been tried to improve the conventional filtration procedure, yet the success rate remains unsatisfactory [4,5]. Cyclodestructive procedures and drainage devices have been tried as an alternative with promising results [4,6,7]. Glaucoma Drainage Devices (GDDs) have recently become a valuable tool for the surgical management of these highly complicated patients [3,4,8,9]. Complications after tube implantation like ‘persistent hypotony’ especially in non valvular devices have discouraged many surgeons to include GDDs in their management strategy but after the introduction of a valvular device with improved safety profile, GDDs have also been tried as primary treatment modality for glaucoma in certain studies [10,11]. All currently available GDDs are based on the concept of the Molteno implant with various modifications such as introduction of a valve mechanism or variations in surface area of the end plate [4,12].
The AGV (New World Medical, Rancho Cucamonga, CA, USA) is a valvular device with silicone elastomers which open at an IOP of 8 mmHg or above and thus decreases the incidence of persistent hypotony that was frequently seen with non valved devices. This improved safety profile that promotes the use of AGV in refractory glaucoma either as a primary surgical option or after failure of conventional filtration procedures [4,13]. The overall success rate varies among different types of refractory glaucomas, ranging from 50% to 80% in different case series with different success criteria and various lengths of follow up [3,4,8,14-17]. The present study was undertaken with a purpose to evaluate the efficacy of AGV in refractory glaucomas in a Government Tertiary Care Centre in North India.
Materials and Methods
A retrospective review of patients that underwent AGV (FP7 or FP9) implantation at the Department of Ophthalmology, Government Medical College and Hospital, Chandigarh, India from January 2012 to December 2014 was performed. Institutional Ethics Committee Approval was taken before undertaking the review. Patients who underwent AGV implantation for refractory glaucoma were evaluated and 30 eyes of 25 patients with a follow-up of at least 12 months were included. Demographic data, included age, sex and preoperative data such as age at the time of the surgery, eye laterality, glaucoma diagnosis, prior ocular history, Best Corrected Visual Acuity (BCVA), IOP and anti-glaucoma medications were noted. Major postoperative complications were also noted. Postoperative data included BCVA, IOP levels, number of medications used, surgical complications, additional surgeries performed, and duration of follow up. Preoperative and postoperative IOPs were measured by Goldmann Applanation Tonometry at every visit.
Surgical Technique
One experienced surgeon performed all surgeries, under monitored anaesthetic care along with peribulbar anaesthesia for adult patients and general anaesthesia for paediatric patients. A clear corneal traction suture using 6-0 prolene was placed parallel to limbus and a limbal based superotemporal peritomy was performed. The AGV (FP7 or FP9) was primed with balanced salt solution, and its plate was secured to the sclera at least 7-8 mm posterior to the surgical limbus with two nylon 9-0 sutures. The tube was trimmed with bevelled anterior surface and inserted into the anterior chamber or the sulcus in a plane parallel to iris and as far from corneal endothelium as possible. A glycerine preserved scleral patch graft 3×3 mm from banked donor sclera was placed over the tube and secured with 10-0 nylon sutures followed by water tight closure of Tenon’s and conjunctiva using 8-0 vicryl sutures. Subconjunctival gentamycin, and dexamethasone were administered at the conclusion of the surgery.
All patients received intensive steroid, antibiotic and cycloplegic drops daily in the postoperative period. The antibiotic drops were stopped at three weeks postoperatively, and steroid drops were tapered gradually over 4-8 weeks. On each follow-up visit all the parameters studied for the postoperative evaluation were documented and decision to start anti-glaucoma medications or to perform other surgeries were taken accordingly.
Statistical Analysis
All statistical analysis were carried out using IBM Statistical Package for Social Sciences (SPSS Version 21.0 for Windows). Descriptive statistics like mean and standard deviation were calculated for all quantitative variables. The main outcome measure was surgical success rate. Success was defined as IOP lower than 21 mmHg and higher than 5 mmHg and at least 30% of IOP reduction with or without glaucoma medications, without additional glaucoma surgery, and a Visual Acuity (VA) of hand motion at least. Kaplan-Meier survival analysis was performed to evaluate success rate over the study period. Repeated measure analysis of variance (ANOVA) along with Bonferroni adjustment was performed to evaluate IOP and medication changes over time. A p-value <0.05 was considered as statistically significant.
Results
Majority of the patients were males (19/25, 76%). Mean age of the patients was 44.90±18.86 (5-80 years). Mean preoperative IOP was 33.47±6.18 mmHg (24-50mmHg). Majority of the eyes were of neovascular glaucoma (n=6, 20%) followed by primary angle closure glaucoma with failed filtration procedure (n=5, 16.7%), secondary open angle glaucoma (n=5, 16.7%), primary open angle glaucoma with failed filtration procedure (n=3, 10%) and post-penetrating keratoplasty glaucoma (n=3, 10%). Overall 20 eyes (67%) had a previous history of failed trabeculectomy, 17 (56.6%) were pseudophakic and 3 (10%) were aphakic [Table/Fig-1].
Demographic profile of the study group.
| Number of eyes (patients) | 30 (25) |
|---|
| Age (years) | Mean±SD | 44.90±18.86 |
| Range | 5-80 |
| Gender (Male/Female) | 19/11 |
| Site of AGV | Supratemporal quadrant, Anterior Chamber | 10 (33.33%) |
| Supratemporal quadrant, Ciliary Sulcus | 20 (66.67%) |
| Glaucoma type | Neovascular Glaucoma | 6 (20%) |
| PACG | 5 (16.7%) |
| Silicon Oil Induced Glaucoma | 5 (16.7%) |
| POAG | 3 (10%) |
| PPKG | 3 (10%) |
| Uveitic Glaucoma | 3 (10%) |
| Angle Recession Glaucoma | 3 (10%) |
| Congenital Glaucoma | 2 (6.6%) |
| Preoperative IOP (mmHg) | Mean±SD | 33.47±6.18 |
| Range | 24-50 |
| Preoperative BCVA (logMAR) | Mean±SD | 1.15±0.93 |
| Range | 0.18-3.0 |
| Preoperative antiglaucoma drugs | Mean±SD | 2.43±1.1 |
| Range | 1-5 |
| Postoperative IOP (mmHg) (p<0.001) | Mean±SD | 12.36±3.55 |
| Range | 10-17 |
| Postoperative BCVA (logMAR) (p=0.83) | Mean±SD | 1.48±1.34 |
| Range | 0.18-4.0 |
| Postoperative Antiglaucoma drugs (p=0.65) | Mean±SD | 1.1±0.5 |
| Range | 0-3 |
| Follow-up period (months) | Mean±SD | 26.8±2.6 |
| Range | 24-36 |
AGV: Ahmed glaucoma valve; IOP: Intraocular pressure; BCVA: Best corrected visual acuity; PACG: Primary angle closure glaucoma; POAG: Primary open angle glaucoma; PPKG: Post penetrating keratoplasty glaucomawas
IOP Changes and Hypertensive Phase
A Hypertensive Phase (HP) has been defined as a rise in IOP to >21 mmHg within three months of AGV implantation, after reduction of IOP to <22 mmHg during the first postoperative week and not caused by tube obstruction, tube retraction, or malfunctioning of the valve [8,18]. Resolution of the HP was defined as an IOP <22 mmHg along with: 1) a reduction of the IOP by 3 mmHg or more with the same number of medications or less; or 2) reduction of at least one medication with a change of IOP <3 mmHg [8,18]. This phase was observed between 1 and 3 months in 11/30 (36.67%) eyes in present study [Table/Fig-2].
Mean intraocular pressure after ahmed glaucoma valve implantation.
The preoperative intraocular pressure of 33.47±6.19 mmHg decreased to 10.14±1.76 mmHg at day one; 15.50±2.47 mmHg at three months (n=30); 16.00±3.96 mmHg (n=30) at six months; 12.30±4.63 mmHg (n=30) at one year and 12.36±3.55 mmHg at three years (n=21) after surgery
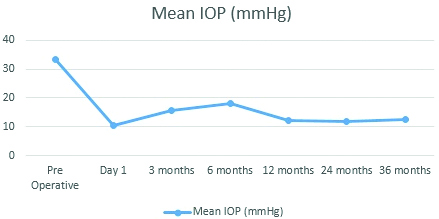
Success of the Implant
The AGV implantation was successful in 90% eyes at 12 months (n=30), 86.7% at 24 months (n=26) and 70% at 36 months (n=21). The Kaplan–Meier life-table analysis showed a cumulative probability of success following AGV implantation as 90% at 1 year and 70% at 3 years. All the patients were previously on anti-glaucoma drugs with 29 eyes (96.7%) on three or more drugs. Postoperatively at 12 months (n=30) 11 eyes (36.7%) did not require any drug, 4 (13.3%) were on one drug and 11 (36.7%) were on 2 drugs. At last follow-up at 36 months (n=21) 6 eyes (28.6%) did not require any drug, 3 (14.3%) were on one drug and 8 (38.1%) were on 2 drugs and 4 (19.0%) required 3 or more drugs.
Complications of the Implant
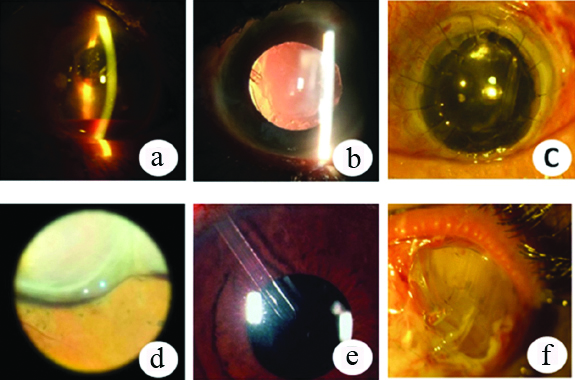
No major intraoperative complications occurred in any of the patients. 7 (23.33%) eyes developed postoperative complications of which, 4 (57.14%) required surgical intervention. Postoperative hyphaema was noted in one eye that was managed conservatively. Two eyes developed increased IOP after tube obstruction with vitreous gel. Nd Yag laser was used to perform vitreolysis of the gel and the IOP was controlled after the procedure. Another eye developed a conjunctival buttonhole for which a conjunctival autograft was applied. There was hypotony (defined as IOP less than 8 mmHg) due to choroidal detachment in one eye which was conservatively managed with oral steroids. Plate exposure occurred in one eye for which conjunctival autograft with amniotic membrane transplant was done twice but ultimately the implant had to be explanted. This patient was detected to have rheumatoid arthritis and was put on treatment by a rheumatologist for the same. In one eye there was corneal decompensation following tube insertion in the anterior chamber. The tube was later trimmed away from the endothelium and subsequently the corneal clarity improved. None of our patients developed a motility disorder, wound leak, bleb related infections or encapsulation [Table/Fig-3,4].
Postoperative complications occurred in seven (23.33%) patients.
| Complication | Management | No of patients |
|---|
| Tube obstruction | Laser Vitreolysis | 2 (6.67%) |
| Hyphaema | Conservative | 1(3.33%) |
| Choroidal detachment | Conservative | 1(3.33%) |
| Tube corneal touch | Surgical | 1(3.33%) |
| Conjunctival buttonhole | Surgical | 1(3.33%) |
| Plate exposure | Failed Surgical, Explant | 1(3.33%) |
a) Hyphaema; b) Vitreous strands occluding the lumen, c) AGV in PPKG, d) Choroidal Detachment, e) Early corneal decompensation, f) Plate exposure requiring AGV explant.
Discussion
The management of refractory glaucomas poses a twin challenge of both difficult diagnosis and complex management. Majority of the eyes require GDD as a primary procedure or secondary surgery after failure of primary filtration surgery. Primary trabeculectomy with antimetabolites and cyclodestructive procedures have also failed to give satisfactory results and often have high incidence of postoperative complications [7,19]. The AGV is a shunt device with a valve to restrict the excessive flow of aqueous humour and has been used in these patients with relative success.
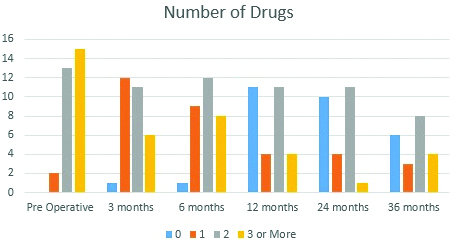
In the present study we retrospectively reviewed the data of patients with refractory glaucoma who underwent AGV implantation at the hospital. AGV reduced the preoperative mean IOP of 33.47±6.19 mmHg to 12.36±3.55 mmHg at last follow-up after surgery (p<0.001) at the end of a mean follow-up period of 26.8±2.6 months. (p=0.76). The dependency on anti-glaucoma drugs decreased from 2.43±1.1 to 1.1±0.5 at the last follow-up visit (p=0.65). The number of anti-glaucoma drugs used at different follow up periods is given in [Table/Fig-5].
Graph showing the number of patients and anti-glaucoma drugs used at different time points.
Note that the number of anti-glaucoma drugs required to control IOP decreases after AGV implantation. At 36 months, 17 eyes required two drugs or less to maintain the IOP
Comparing success rates from different published studies was hazardous because different studies have different study designs, different types of implants and different demographics. Most of the published studies evaluating role of AGV in refractory glaucomas in Indian eyes report encouraging success rates [8,14,15,17]. Present study shows a similar success rate of 70% at the end of a follow-up period of 36 months. Previously, a study from North India has reported a success rate of 85.45% at 1 year and 79.63% at 3 years which is comparable to present success rate of 90% at 1 year and 70% at 3 years [3,8,14-16].
The incidence of postoperative complications in present study was 23.33% which is comparative to the data published previously [8,14,15]. Dubey S et al., reported an incidence of 25.45% out of which postoperative hypotony occurred in 10.90% of the patients [8]. In present study one patient developed postoperative hypotony due to choroidal detachment and it resolved on giving oral steroids.
We did not encounter any case of encapsulation in present study. Previously Parihar JK et al., have reported as high as 12% encapsulated blebs in their study that required needle puncture [14]. Dubey S et al., do not report any case of encapsulated blebs in their study. They used silicone AGV in most of the eyes [8]. Implant endplate size and its material have been previously shown to affect implant success rate and need of additional surgical intervention [20-22]. These observations indicate that use of silicone based implants can reduce the incidence of bleb encapsulation and subsequent interventions. In present study we used silicone AGV (FP7 and FP9) in all of the eyes.
Hypertensive phase was seen in 36.67% of present study population. This is higher than the 27.27% reported by Dubey S et al., [8]. The Present patients were treated conservatively with anti-glaucoma medications and all eyes were controlled with medications without need of any surgical intervention. The hypertensive phase was more commonly seen in AGV implant than other non valvular GDDs and higher preoperative IOP and myopia have been shown as risk factors for this phenomenon [23,24]. We agree with the observation made by that silicone based implants have lesser tendency to show the hypertensive phase which was reported in as high as 80% eyes in previous studies [8,15,22,25].
The plate of the implant is routinely placed posteriorly around 7-8 mm from the limbus so as to promote the formation of filtering bleb posterior to the equator. The encapsulation around the plate offers resistance to outflow of aqueous. The patients having hypertensive phase have more avascular bleb and have well defined edges [4]. Newer imaging techniques like AS OCT can be employed to evaluate bleb morphology and detect changes that can be then dealt with at the appropriate time and thus avoid implant failure at a later stage [26].
Vitreous incarceration of the tube lumen was a common complication noted post GDD implantation and was found in two patients and was managed using Nd YAG laser without the need of surgical intervention [27]. Anterior vitrectomy at the time of GDD implantation can potentially prevent vitreous incarceration of the tube lumen.
Tube or plate exposure is one the most feared complication post GDD implantation and requires surgical intervention with a wide variety of patch graft materials and repair techniques [28]. In present case we had plate exposure in a patient of rheumatoid arthritis that required explantation of the implant. Repair was done using scleral patch graft and amniotic membrane but both times the tissue melted away despite systemic and topical medications. The conjunctival swab, tissue biopsy and explanted implant were all sterile on microbiological examination. This patient was detected to have elevated levels of RA factor and was diagnosed with rheumatoid arthritis and subsequently referred to a rheumatologist for further management. We could not find any other similar report that describes the management of AGV plate exposure in a patient with refractory rheumatoid arthritis. In an opinion it is imperative to evaluate the systemic status of the patient before planning AGV implantation so as to avoid such complications later in the postoperative period.
The GDD’s and their effect on the cornea have been studied previously. Factors like inflammation, aqueous humour dynamics, micro motion of the tube and anterior migration have been elucidated as risk factors for endothelial cell loss and subsequent corneal decompensation [29]. Surgical intervention has been frequently described in previously published studies for corneal decompensation [16,30,31]. In present study one eye developed corneal decompensation two weeks after surgery and simple trimming of the tube resulted in improvement of the corneal clarity. Previously studies have also described other techniques that can be used to manipulate the tube away from the cornea and prevent the need of keratoplasty [31-33].
Limitation
The retrospective design and small sample size are the main limitations of present study. Secondly, we had unequal number of patients of different types of glaucoma and thus could not compare the success rate of AGV implantation and risk factors for failure in various groups.
Conclusion
In conclusion present study supports the expanding use of AGV in management of refractory glaucomas. The AGV implant offers good IOP control and freedom from anti-glaucoma medications while maintaining a reliable safety profile. Further studies are however needed to evaluate the use of GDD’s as primary procedure especially in cases where conventional glaucoma filtration surgery has high chances of failure.
AGV: Ahmed glaucoma valve; IOP: Intraocular pressure; BCVA: Best corrected visual acuity; PACG: Primary angle closure glaucoma; POAG: Primary open angle glaucoma; PPKG: Post penetrating keratoplasty glaucomawas
[1]. Nassiri N, Kamali G, Rahnavardi M, Mohammadi S, Nassiri S, L Rahmani, Ahmed glaucoma valve and single-plate Molteno implants in treatment of refractory glaucoma: a comparative studyAm J Ophthalmol 2010 149(6):893-902.10.1016/j.ajo.2010.01.02520451896 [Google Scholar] [CrossRef] [PubMed]
[2]. Nguyen QH, Primary surgical management refractory glaucoma: tubes as initial surgeryCurr Opin Ophthalmol 2009 20(2):122-25.10.1097/ICU.0b013e32831da82819240544 [Google Scholar] [CrossRef] [PubMed]
[3]. Souza C, Tran DH, Loman J, Law SK, Coleman AL, Caprioli J, Long-term outcomes of Ahmed glaucoma valve implantation in refractory glaucomasAm J Ophthalmol 2007 144(6):893-900.10.1016/j.ajo.2007.07.03517916318 [Google Scholar] [CrossRef] [PubMed]
[4]. Riva I, Roberti G, Katsanos A, Oddone F, Quaranta L, A review of the Ahmed glaucoma valve implant and comparison with other surgical operationsAdv Ther 2017 34(4):834-47.10.1007/s12325-017-0503-128283892 [Google Scholar] [CrossRef] [PubMed]
[5]. Aref AA, Gedde SJ, Budenz DL, Glaucoma drainage implant surgeryDev Ophthalmol 2017 59:43-52.10.1159/00045848528442686 [Google Scholar] [CrossRef] [PubMed]
[6]. Aquino MC, Barton K, Tan AM, Sng C, Li X, Loon C, Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: a randomized exploratory studyClin Exp Ophthalmol 2015 43(1):40-46.10.1111/ceo.1236024811050 [Google Scholar] [CrossRef] [PubMed]
[7]. Bloom PA, Tsai JC, Sharma K, Miller MH, Rice NS, Hitchings RA, "Cyclodiode". Trans-scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucomaOphthalmology 1997 104(9):1508-19.10.1016/S0161-6420(97)30109-2 [Google Scholar] [CrossRef]
[8]. Dubey S, Sharma V, Agrawal A, Chauhan L, Douglas G, Safety and efficacy of Ahmed glaucoma valve implantation in refractory glaucomas in Northern Indian eyesSaudi J Ophthalmol 2015 29(2):103-08.10.1016/j.sjopt.2014.06.00727616909 [Google Scholar] [CrossRef] [PubMed]
[9]. Shen CC, Salim S, Du H, Netland PA, Trabeculectomy versus Ahmed Glaucoma Valve implantation in neovascular glaucomaClin Ophthalmol 2011 5:281-86.10.2147/OPTH.S1697621468334 [Google Scholar] [CrossRef] [PubMed]
[10]. Molteno AC, Bevin TH, Herbison P, Husni MA, Long-term results of primary trabeculectomies and Molteno implants for primary open-angle glaucomaArch Ophthalmol 2011 129(11):1444-50.10.1001/archophthalmol.2011.22121746973 [Google Scholar] [CrossRef] [PubMed]
[11]. Gedde SJ, Singh K, Schiffman JC, Feuer WJ, The Tube Versus Trabeculectomy Study: interpretation of results and application to clinical practiceCurr Opin Ophthalmol 2012 23(2):118-26.10.1097/ICU.0b013e32834ff2d122249235 [Google Scholar] [CrossRef] [PubMed]
[12]. Molteno AC, New implant for drainage in glaucomaClinical trial. Br J Ophthalmol 1969 53(9):606-15.10.1136/bjo.53.9.6064900144 [Google Scholar] [CrossRef] [PubMed]
[13]. Coleman AL, Hill R, Wilson MR, Choplin N, R Kotas-Neumann, Tam M, Initial clinical experience with the Ahmed Glaucoma Valve implantAm J Ophthalmol 1995 120(1):23-31.10.1016/S0002-9394(14)73755-9 [Google Scholar] [CrossRef]
[14]. Parihar JK, Vats DP, Maggon R, Mathur V, A Singh, Mishra SK, he efficacy of Ahmed glaucoma valve drainage devices in cases of adult refractory glaucoma in Indian eyes.T Indian J Ophthalmol 2009 57(5):345-50.10.4103/0301-4738.5506819700871 [Google Scholar] [CrossRef] [PubMed]
[15]. Panda A, Prakash VJ, Dada T, Gupta AK, S Khokhar, Vanathi M, Ahmed glaucoma valve in post-penetrating-keratoplasty glaucoma: a critically evaluated prospective clinical studyIndian J Ophthalmol 2011 59(3):185-89.10.4103/0301-4738.8102121586837 [Google Scholar] [CrossRef] [PubMed]
[16]. Zarei R, Amini H, Daneshvar R, Nabi F, S Moghimi, Fakhraee G, Long-term Outcomes of Ahmed Glaucoma Valve Implantation in Refractory Glaucoma at Farabi Eye Hospital, Tehran, IranMiddle East African Journal of Ophthalmology 2016 23(1)(1):104-09.10.4103/0974-9233.16461126957848 [Google Scholar] [CrossRef] [PubMed]
[17]. Das JC, Chaudhuri Z, Sharma P, Bhomaj S, The Ahmed Glaucoma Valve in refractory glaucoma: experiences in Indian eyesEye (Lond) 2005 19(2):183-90.10.1038/sj.eye.670144715258600 [Google Scholar] [CrossRef] [PubMed]
[18]. Nouri-Mahdavi K, Caprioli J, Evaluation of the hypertensive phase after insertion of the Ahmed Glaucoma ValveAm J Ophthalmol 2003 136(6):1001-08.10.1016/S0002-9394(03)00630-5 [Google Scholar] [CrossRef]
[19]. Nakatake S, Yoshida S, Nakao S, Arita R, Yasuda M, Kita T, Hyphema is a risk factor for failure of trabeculectomy in neovascular glaucoma: a retrospective analysisBMC Ophthalmology 2014 14:5510.1186/1471-2415-14-5524766841 [Google Scholar] [CrossRef] [PubMed]
[20]. Hinkle DM, Zurakowski D, Ayyala RS, A comparison of the polypropylene plate Ahmed glaucoma valve to the silicone plate Ahmed glaucoma flexible valveEur J Ophthalmol 2007 17(5):696-701.10.1177/11206721070170050217932842 [Google Scholar] [CrossRef] [PubMed]
[21]. Bai YJ, Li YQ, Chai F, Yang XJ, Zhang YC, Wei YT, omparison of FP-7 and S-2 Ahmed glaucoma valve implantation in refractory glaucoma patients for short-term follow-upC. Chin Med J (Engl) 2011 124(8):1128-33. [Google Scholar]
[22]. Ishida K, Netland P, Costa VP, Shiroma L, Khan B, Ahmed I, Comparison of Polypropylene and Silicone Ahmed Glaucoma ValvesOphthalmology 2006 113(8):1320-26.10.1016/j.ophtha.2006.04.02016877071 [Google Scholar] [CrossRef] [PubMed]
[23]. Jung KI, Park CK, Risk factors for the hypertensive phase after implantation of a glaucoma drainage deviceActa Ophthalmol 2016 94(5):e260-67.10.1111/aos.1291626603240 [Google Scholar] [CrossRef] [PubMed]
[24]. Won HJ, Sung KR, Hypertensive phase following silicone plate ahmed glaucoma valve implantationJ Glaucoma 2016 25(4):e313-17.10.1097/IJG.000000000000024925774945 [Google Scholar] [CrossRef] [PubMed]
[25]. Ayyala RS, Zurakowski D, Monshizadeh R, Hong CH, Richards D, Layden WE, Comparison of double-plate Molteno and Ahmed glaucoma valve in patients with advanced uncontrolled glaucomaOphthalmic Surg Lasers 2002 33(2):94-101. [Google Scholar]
[26]. Jung KI, Park H, Jung Y, Park CK, Serial changes in the bleb wall after glaucoma drainage implant surgery: characteristics during the hypertensive phaseActa Ophthalmol 2015 93(4):e248-53.10.1111/aos.1257125363490 [Google Scholar] [CrossRef] [PubMed]
[27]. Desatnik HR, Foster RE, Rockwood EJ, Baerveldt G, Meyers SM, Lewis H, Management of glaucoma implants occluded by vitreous incarcerationJ Glaucoma 2000 9(4):311-16.10.1097/00061198-200008000-0000510958604 [Google Scholar] [CrossRef] [PubMed]
[28]. Thakur S, Ichhpujani P, Kumar S, Grafts in glaucoma surgery: a review of the literatureAsia Pac J Ophthalmol (Phila) 2017 6(5):469-76. [Google Scholar]
[29]. Koo EB, Hou J, Keenan JD, Stamper RL, Jeng BH, Han Y, Effects of glaucoma tube surgery on corneal endothelial cells: a reviewEye Contact Lens 2016 42(4):221-24.10.1097/ICL.000000000000017126222096 [Google Scholar] [CrossRef] [PubMed]
[30]. Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC, Surgical complications in the Tube Versus Trabeculectomy Study during the first year of follow-upAm J Ophthalmol 2007 143(1):23-31.10.1016/j.ajo.2006.07.02217054896 [Google Scholar] [CrossRef] [PubMed]
[31]. Bochmann F, Azuara-Blanco A, Transcameral suture to prevent tube-corneal touch after glaucoma drainage device implantation: a new surgical techniqueJ Glaucoma 2009 18(8):576-77.10.1097/IJG.0b013e318191128419826384 [Google Scholar] [CrossRef] [PubMed]
[32]. Bersudsky V, Treviño A, Rumelt S, Management of endothelial decompensation because of glaucoma shunt tube touch by Descemet membrane endothelial keratoplasty and tube revisionCornea 2011 30(6):709-11.10.1097/ICO.0b013e3181fb837821242787 [Google Scholar] [CrossRef] [PubMed]
[33]. Ma KT, Kim JH, Seong GJ, Jang DS, Kim CY, Scleral fixation of Ahmed glaucoma valve tube tip for adjustment of cornea-touching malpositionEye 2014 28(1):23-25.10.1038/eye.2013.21424097119 [Google Scholar] [CrossRef] [PubMed]