Introduction
Diabetes mellitus is a group of metabolic diseases characterised by hyperglycaemia arising as a consequence of a relative or absolute deficiency of insulin secretion, resistance to insulin action or both. Pre-diabetes as defined by American Diabetes Association (ADA) includes individuals who have impaired Fasting Plasma Glucose (FPG 100-125 mg/dL) and/or Impaired Glucose Tolerance (IGT) where in plasma glucose level varies from 140-199 mg/dL during OGTT [1]. Obesity increases the risk of multiple metabolic diseases such as hyperlipidemia, Type 2 diabetes mellitus, atherosclerosis and cardiovascular complications etc., [2]. The perspective of adipose tissue as merely a fat depot has changed in recent times. Focus is now being laid on the metabolic and inflammatory functions of adipose tissue, which is modulated through a variety of bioactive mediators, called adipokines like leptin, adiponectin, TNF-α, interleukin-6 etc., [3]. Omentin is a newly identified adipokine isolated from the visceral omental adipose tissue. Whereas, omentin m-RNA is barely detectable in the subcutaneous fat depot, it has been identified in other tissues like in intestinal paneth cells and endothelial cells at lower expression levels [4,5]. A homolog of omentin has been identified which bears 83% amino acid homology with omentin and is referred to as omentin-2. Omentin-2 is expressed in considerable higher levels in the intestine and in very low levels in visceral fat. Omentin discovered from visceral fat is now being referred to as omentin-1, which is also the major circulating form, whereas omentin-2 is not detectable in plasma. The two omentin genes are localised adjacent to each other in the 1q22-q23 chromosomal region which has been previously linked to Type 2 diabetes [6-8]. The insulin signal transduction is increased by omentin-1 by activating protein kinase AKT/protein kinase B. The effect of Omentin-1 on AKT signaling is both in presence or absence of insulin [4]. It also enhances insulin stimulated glucose transport in isolated human adipocytes, thus omentin may play a role in the modulation of insulin sensitivity [7].
Metabolic disturbances associated with obesity are also associated with inflammation. Cross sectional studies on humans with Normal Glucose Tolerance (NGT) and IGT, for association between vascular function and omentin, have suggested that obesity associated metabolic dysfunctions and pro inflammatory cytokines such as IL-6 and CRP are associated with omentin levels. Omentin influenced the variance of endothelium dependent vasodilatation when controls were put in place for age, obesity and inflammation [9]. The effect on endothelium by omentin is via suppression of adhesion of monocytes to TNF alpha activated endothelial cells and via interruption of ICAM-1 and VACAM-1 expression by inhibition of NF-kb signaling pathway [10,11]. Omentin-1 levels are decreased in obesity and diabetes mellitus [7,8,12-14]. Decreased levels are observed in overweight and also in non obese insulin resistant female patients with polycystic ovary syndrome [15,16]. Circulating omentin-1 has been also suggested to play a beneficial role in preventing atherosclerosis and has cardio protective effect [17-19]. Weight reduction has been shown to result in increase in circulating omentin-1 levels and recent studies have shown that its plasma levels correlate negatively with obesity and insulin resistance and positively with adiponectin and HDL-C [8,12,13,20-22].
To the best of our knowledge, plasma omentin-1 levels have not been evaluated in obese prediabetic subjects in India. In view of the preferential expression of omentin-1 in visceral fat, its presence in circulation and its potential role as insulin sensitiser; present study was planned: a) to evaluate plasma omentin-1 levels in obese prediabetic, obese normoglycaemic and healthy individuals; and b) to explore how omentin levels correlate with measures of obesity and insulin resistance.
Materials and Methods
This observational case control study was conducted at University College of Medical Sciences (UCMS) and GTB Hospital, Delhi, India during the duration of November 2009 to May 2012, on 150 males of age group 20-50 years. Based on sample sizes used in the comparable studies, it was decided that 50-50-50 sample size would be optimal for present study [12,13]. Only males were included in the study as the levels of omentin-1 differ in males and females [10]. So, this selection brought homogeneity, and avoided variations of omentin-1 levels. All subjects were enrolled from “Diabetic Clinic” and Medicine OPD of the UCMS and GTB Hospital. The institute statistician suggested a sample size of 50 each for three groups. The Oral Glucose tolerance (OGTT) was performed in overnight fasted (10 to 12 hours) subjects. Fasting blood samples were collected and they were given 75 g of glucose in 250 mL of water. Blood samples were collected again after 120 minutes of glucose load. Based on the results of anthropometrical measurements and OGTT test, three groups of 50 males each (20- 50 years) were selected.
They were selected consecutively and divided into following groups:
Group-1 (Healthy controls): Consisted of 50 healthy males with Waist Circumference (WC) less than 90 cm having NGT (2 hour OGTT <140 mg/dL) and normal FPG (i.e., FPG <100 mg/dL).
Group-2 (Obese normoglycaemic, Obese NGT): 50 obese males with waist circumference ≥90 cm having NGT and normal FPG.
Group-3 (Obese pre-diabetic, Obese PD): 50 obese males with waist circumference ≥90 cm (and having IGT and/or Impaired Fasting Glucose (IFG).
Classification of study groups as prediabetics, normoglycaemic and healthy control was done according to ADA criteria [1]. For central obesity, IDF criterion was used, as discussed under anthropometry [23].
Ethical clearance for the study was obtained from the Institutional Ethical Committee on human research and Informed consent was taken from all the individual participants included in the study. Subjects having malignant disease, major renal, hepatic and/or thyroid dysfunctions were excluded from the study. The age, family history and physical examination of all the subjects were systematically recorded in a pre-designed proforma and a detailed clinical history was taken.
Anthropometric Measurements
Body Mass Index (BMI) was calculated as body weight in kilograms divided by square of height in meters (kg/m2), WC was measured using a non-stretchable measuring tape midway between the inferior margin of the ribs and the superior border of the iliac crest during mid expiration, the subjects were asked to stand erect in a relaxed position with both feet together on a flat surface with one layer of clothing. The subject’s hip circumference was measured as the maximum circumference around the buttocks posteriorly at the level of greater trochanters (hip bones) and measured in centimeters.
Subjects with WC ≥90 cm were considered as having central obesity. According to revised WHO criteria the cut off value for BMI and waist circumference for overweight/obese and centrally obese in South Asian males is ≤23 kg/m2 and 90 cm respectively (WHO, International Diabetic Federation) [23]. Therefore, we have considered males of BMI ≤23 kg/m2 as overweight/obese and waist circumference ≤90cm as centrally obese.
Biochemical Investigations
Fasting blood samples (10 hours) were collected with an aseptic blood collection technique by the use of sterile gloves and through disinfection of venepuncture site with 70% ethyl alcohol. Samples were collected in sitting position and were centrifuged within one hour at 1500 rpm for 15 minute. These were processed to obtain FPG, lipid profile, HbA1c, fasting insulin and Omentin-1. Subjects were then asked to drink solution of 75 g of glucose in 250 mL of water and after two hours blood sample were drawn for Postprandial Plasma Glucose (PPG) test. FPG, PPG were estimated by glucose oxidase method [24]. Total Cholesterol (TC), High-Density Lipoprotein Cholesterol (HDL-C), and Triacylglycerol (TAG) were determined using kits from Accurex Biomedical Pvt. Ltd., Mumbai, India. Very Low Density Lipoprotein Cholesterol (VLDL-C) was calculated as value of TAG divided by five. Low-Density Lipoprotein Cholesterol (LDL-C) was calculated from the Friedwald and Fredrickson’s formula [25].
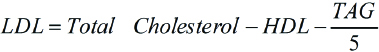
According to National Cholesterol Education Program- Adult Treatment Panel (NCEP ATP) III guidelines, LDL Cholesterol of <100 mg/dL is considered optimal, Total cholesterol of <200 mg/dL is considered desirable and HDL Cholesterol of <40 mg/dL is considered as low [26].
Glycated haemoglobin was determined by ion-exchange resin method, using kits from Erba Diagnostics [27]. Individuals with HbA1c of <5.7% are considered normal. Those with HbA1c values from 5.7-6.4% are considered prediabetics where as those with values >6.5 are considered diabetic [1]. Insulin was estimated in plasma by ELISA kits from Mercodia. The normal value of fasting serum insulin is <25 mIU/L [28]. Plasma omentin-1 was estimated by ELISA using kits from BioVendor, Germany.
Principle and Methodology of Estimation of Omentin-1
Samples of serum were incubated in microtitration wells coated with polyclonal anti-human omentin-1 antibody. After a thorough wash, the biotin- labeled polyclonal anti-human omentin-1 antibody was added and incubated with captured omentin-1, after another washing streptavidin- HRP conjugate was added. After another wash the remaining conjugate was allowed to react with the substrate solution (TMB). The reaction was stopped by addition of acidic solution absorbance of the resulting yellow color product was measured spectrophotometrically at 450 nm. The absorbance was proportional to the omentin-1 concentration. A standard curve was constructed by plotting absorbance values versus concentrations of serum omentin-1 standard, and the concentrations of unknown samples were determined (ng/mL) using this standard curve.
Insulin resistance was estimated as Homeostatic Model Assessment of Insulin Resistance index (HOMA-IR) [29]. It assesses beta cell function and IR from fasting glucose and insulin or C-peptide concentrations. There is a feedback loop between the hepaticglucose output and beta cells of pancreas, which governs the relationship between glucose and insulin in the basal state [30].
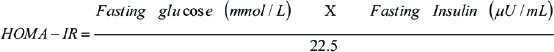
The denominator of 22.5 is a normalising factor; i.e., the product of normal fasting plasma insulin of 5 U/mL and normal fasting plasma glucose of 4.5 mmol/L; typical of a “normal” healthy individual is 22.5. Therefore, for an individual with “normal” insulin sensitivity, HOMA-IR will be one [31].
Statistical Analysis
The sample size of 150 subjects was taken arbitrarily. All the data required for the study was collected and analysed statistically to determine the significance of different parameters by using statistical software IBM SPSS Statistics, version 17.0 software (IBM Corp, New York). All values were described as Mean±Standard Deviation (SD) values. Statistical analysis was carried out by one-way ANOVA followed by Tukey’s test at 5% level. Pearson’s correlation coefficient was used for calculation of association of variables. A p-value less than 0.05 was considered as significant (S) and less than 0.01 Highly Significant (HS).
Results
The base line characteristics of the study subjects are shown in [Table/Fig-1]. BMI, waist circumferences and Waist to Hip (W/H) ratio were significantly higher (p<0.001) in obese NGT and obese pre diabetic groups as compared to healthy controls. Systolic and Diastolic Blood Pressure (SBP, DBP) were within normal range in all the groups.
Anthropometric data of obese pre-diabetic, obese normoglycaemic and healthy controls.
| Variables | Group-1 (healthy controls, n=50) (mean±SD) | Group-2 (obese normoglycaemic,n=50) (mean±SD) | Group-3 (obese pre-diabetic, n=50) (mean±SD) |
|---|
| Age (years) | 41.7±9.20 | 37.9±7.30 | 43.5±7.60 |
| BMI (kg/m2) | 19.98±2.25 | 26.12±3.08 a | 27.23±3.11 bc |
| Waist circumference (WC) (cm) | 71.77±6.71 | 95.00±4.57 a | 97.53±4.38 bc |
| Waist To Hip Ratio (WHR) | 0.86±0.04 | 0.93±0.07 a | 0.97±0.06 bc |
| Systolic Blood Pressure (mmHg) | 107.53±9.80 | 119.1±15.31 | 124.6±19.67 |
| Diastolic Blood Pressure (mmHg) | 67.6±4.01 | 77.5±8.75 | 80.1±10.15 |
a): Significant difference between values in Group 2 and Group 1; b): Significant difference between values in Group 3 and Group 1; c): Significant difference between values in Group 3 and Group 2; p<0.05 is considered significant
Biochemical parameters: Fasting and postprandial blood glucose and HbA1c values were similar in healthy controls and obese NGT group, while value of these parameters was significantly higher in obese PD group (p<0.001) [Table/Fig-2]. Fasting serum insulin was higher in obese NGT and obese PD groups as compared to healthy control group. Insulin resistance as estimated by HOMA-IR was higher in obese PD group in comparison to other two groups. Plasma omentin-1 levels were significantly lower (p<0.001) in obese NGT (298.4±79.8 ng/mL) and obese PD (228.7±13.4 ng/mL) groups as compared to healthy controls (408.0±98.9 ng/mL). Obese PD group showed significantly lower levels of plasma omentin-1 as compared to obese NGT [Table/Fig-2]. Total cholesterol levels were significantly higher (p<0.001) in obese PD and obese NGT groups compared to healthy controls. Both groups had total cholesterol above 200 mg/dL, which is the upper limit of accepted level of total cholesterol by National Cholesterol Education Programs [26]. HDL-C was significantly lower in obese PD group compared to other two groups. LDL-C and TAG were significantly higher in obese PD group compared to other two groups. TAG was well above accepted level as 150 mg/dL in both obese groups [Table/Fig-3].
Fasting and postprandial plasma glucose and glycosylated haemoglobin, fasting serum insulin, omentin-1 and HOMA-IR in the study subjects (n=50).
| Variables | Group-1 (healthy controls, n=50) (mean±SD) | Group-2 (obese normoglycaemic,n=50) (mean±SD) | Group-3 (obese pre-diabetic, n=50) (mean±SD) |
|---|
| Plasma glucose fasting (mg/dL) | 82.3±8.10 | 80.6±7.00 | 99.4±22.80 bc |
| Plasma glucose postprandial (mg/dL) | 98.7±15.30 | 100.9±20.80 | 144.3±5.10 bc |
| Hb A1c (%) | 5.31±0.49 | 5.4±0.34 | 5.9±0.50 bc |
| Fasting Serum Insulin (μU/mL) | 5.5±2.90 | 8.4±4.78 a | 11.2±3.20 bc |
| Omentin-1 (ng/mL) | 408.0±98.9 | 298.4±79.8 a | 228.7±13.4 bc |
| HOMA-IR (homeostatic model of insulin resistance) | 1.10±0.58 | 1.66±0.96 a | 2.25±1.20 bc |
a): Significant difference between values in Group 2 and Group 1; b): Significant difference between values in Group 3 and Group 1; c): Significant difference between values in Group 3 and Group 2; p<0.05 is considered significant
Lipid profile parameters in obese pre-diabetic, obese normo-glycaemic and healthy controls.
| Variables | Group-1 (healthy controls, n=50) (mean±SD) | Group-2 (obese normoglycaemic,n=50) (mean±SD) | Group-3 (obese pre-diabetic, n=50) (mean±SD) |
|---|
| Total Cholesterol (mg/dL) | 174.5±25.1 | 212.4±34.6 a | 239.7±26.3 bc |
| HDL Cholesterol (mg/dL) | 46.7±4.3 | 42.1±4.1 | 37.3±3.7 bc |
| LDL Cholesterol (mg/dL) | 103.1±13.4 | 138.7±34.2 a | 165.3±25.4 bc |
| VLDL Cholesterol (mg/dL) | 21.7±3.9 | 31.6±4.9 a | 35.1±5.7 bc |
| Triacylglycerol (mg/dL) | 108.5±19.6 | 157.8±24.7 a | 175.6±28.8 bc |
a): Significant difference between values in Group 2 and Group 1; b): Significant difference between values in Group 3 and Group 1; c): Significant difference between values in Group 3 and Group 2; p<0.05 was considered significant; One-way ANOVA followed by Tukey’s test at 5% level
Correlation studies indicates that plasma omentin-1 has negative and highly significant correlation (p<0.001) with BMI, WC, WHRs, PPG, HbA1c, total cholesterol, LDL-C, VLDL-C and TAG. It has a negative and significant correlation with FBG (p=0.002), fasting serum insulin (p=0.014), and HOMA-IR (p=0.009). It has positive and significant correlation (p=0.002) with HDL-C [Table/Fig-4].
Correlation of omentin-1 with markers of obesity, lipid profile, plasma glucose, serum insulin, and insulin resistance marker, HOMA-IR (n=150).
| Variables | Omentin-1 (ng/mL) |
|---|
| Pearson cor-relation coefficient (r-value) | p-value |
|---|
| BMI (kg/m2) | -0.543 | <0.001 (HS) |
| Waist Circumference(cm) | -0.565 | <0.001 (HS) |
| Waist hip ratio | -0.520 | <0.001 (HS) |
| Plasma Glucose Fasting (mg/dL) | -0.322 | <0.002 (S) |
| Plasma Glucose Postprandial (mg/dL) | -0.448 | <0.001 (HS) |
| Glycosylated Haemoglobin HbA1c (%) | -0.376 | <0.001 (HS) |
| Total-Cholesterol (mg/dL) | -0.411 | <0.001 (HS) |
| HDL-Cholesterol (mg/dL) | 0.365 | <0.002 (S) |
| LDL-Cholesterol (mg/dL) | -0.375 | <0.001 (HS) |
| VLDL-Cholesterol (mg/dL) | -0.542 | <0.001 (HS) |
| Triacylglycerol (mg/dL) | -0.542 | <0.001 (HS) |
| Serum fasting Insulin (μU/mL) | -0.258 | 0.014 (S) |
| HOMA-IR | -0.364 | 0.009 (S) |
Pearson’s cor-relation coefficient was used for calculation of association of variables. (p<0.05 was considered significant; p<0.01 was considered highly significant) HS =Highly Significant; S =Significant); HOMA-IR: Homeostatic model of insulin resistance
Discussion
The pioneer ICMR-INDIAB study shows the prevalence of prediabetes in India between 8-15 % in different states. It estimated that India may have 62.4 million diabetic population and 77.2 million prediabetics by 2011 [32]. The ICMR-INDIAB study also demonstrated an alarming rate of dyslipidemia and abdominal obesity along with their correlation [33,34]. We have observed decreased plasma omentin-1 levels in obese NGT subjects as demonstrated in previous studies [4-8]. Important finding of the present study was that plasma omentin-1 levels in obese Prediabetics (PD) patients were significantly decreased compared to obese NGT group (p<0.004). Plasma omentin-1 levels have been rarely studied in prediabetics, while it has not been previously reported in Indian obese PD patients [8]. The exact factors contributing to markedly reduced circulating omentin-1 levels in obese NGT and obese PD subjects still remain to be determined. Moreover, both of these groups also had increased serum insulin levels, which might be an important contributor preceding decreased omentin-1 levels. Decreased plasma omentin-1 has also been reported in obese and non obese females with polycystic ovary syndrome who have increased serum insulin levels [15,16]. It may be possible that insulin regulates synthesis of omentin, directly or indirectly.
Another possible player contributing to decreased omentin-1 levels could be adiposity and obesity associated metabolic complications. This possibility is supported by the finding that circulating omentin-1 levels were increased in patient with anorexia nervosa with severely reduced body fat mass [35]. Obese PD patients had significantly increased plasma glucose and serum insulin levels compared to obese NGT group which may have contributed to further decrease in plasma omentin-1 levels in this group. Tan BK et al., demonstrated that insulin and glucose significantly decreased omentin-1 mRNA expression and omentin-1 protein production in omental adipose tissue explants in dose dependent manner, and further demonstrated that hyperinsulinemia significantly reduced plasma omentin-1 levels even in healthy subjects [15].
Both obese NGT and obese PD groups have significantly increased BMI, WC, and WHR. Patients with BMI more than >23 kg/m2 were considered obese/overweight and patients with WC >90 cm as centrally obese as recommended by WHO and international diabetes federation for South Asian males. Since, omentin-1 level in females is different than males, only male subjects were included in the study. Prediabetic patients in the present study included 20 patients with IFG and 30 patients with IGT. People with prediabetes are at increased risk of developing overt diabetes. Present results for HbA1c for obese PD are in agreement with results of Pan HY et al., for similar group [13]. We observed dyslipidemia in both obese NGT and obese PD groups. Levels of total cholesterol, LDL-cholesterol and triacylglycerol were significantly high and HDL-C significantly low in both of these groups compared to healthy controls.
An important observation of present study is that, there was a negative and significant correlation of plasma omentin-1 with BMI, WHR, FBG, PPG, fasting insulin, TAG and HOMA-IR, whereas omentin-1 has a significant and positive correlation with HDL cholesterol. Positive correlation of omentin-1 with HDL-cholesterol has been previously described in obesity, metabolic syndrome, diabetes and cardiovascular disease [8,12,35,36]. However, mechanisms underlying this relation are less clear. A possible explanation is that disregulation of omentin-1 may adversely affect insulin signaling and regulation of lipoprotein metabolism by insulin and thereby altering HDL production. A similar situation has been described for the adipokine adiponectin [37].
Limitation
As the study was a part of MD Postgraduate thesis, which is a funding and time constraint activity, the sample size was taken as 150 only (with 50 subjects in three groups each). Further studies with a bigger sample size are recommended.
Conclusion
There is significant decrease in the levels of plasma omentin-1 and increase in HOMA-IR marker of insulin resistance in obese PD patients compared to obese normoglycaemic and healthy controls. Plasma omentin-1 has negative and significant correlation with HOMA-IR. As omentin-1 increases sensitivity to insulin, its decreased levels in obese prediabetic patients may be responsible for impaired glucose homeostasis.
Compliance with Ethical Standards
Ethical approval: The study was approved by the Institutional Ethics Committee, University College of Medical Sciences, UCMS, Delhi. All the study procedures were conducted in accordance with the ethical standards of the Institutional Ethics Committee and national research committee (CDSCO) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was taken from all the individual participants included in the study.
a): Significant difference between values in Group 2 and Group 1; b): Significant difference between values in Group 3 and Group 1; c): Significant difference between values in Group 3 and Group 2; p<0.05 is considered significant
a): Significant difference between values in Group 2 and Group 1; b): Significant difference between values in Group 3 and Group 1; c): Significant difference between values in Group 3 and Group 2; p<0.05 is considered significant
a): Significant difference between values in Group 2 and Group 1; b): Significant difference between values in Group 3 and Group 1; c): Significant difference between values in Group 3 and Group 2; p<0.05 was considered significant; One-way ANOVA followed by Tukey’s test at 5% level
Pearson’s cor-relation coefficient was used for calculation of association of variables. (p<0.05 was considered significant; p<0.01 was considered highly significant) HS =Highly Significant; S =Significant); HOMA-IR: Homeostatic model of insulin resistance
[1]. American Diabetes Association. Diagnosis and classification of diabetes mellitusDiabetes Care 2014 37:S81-90.10.2337/dc14-S08124357215 [Google Scholar] [CrossRef] [PubMed]
[2]. Bergman RN, Kim SP, Hsu IR, Catalano KJ, Chiu JD, Kabir M, Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular riskAm J Med 2007 120(suppl 1):S3-8.10.1016/j.amjmed.2006.11.01217296343 [Google Scholar] [CrossRef] [PubMed]
[3]. Blüher M, Adipose tissue dysfunction in obesityExp Clin Endocrinol Diabetes 2009 117(6):241-50.10.1055/s-0029-119204419358089 [Google Scholar] [CrossRef] [PubMed]
[4]. Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin actionAm J Physiol Endocrinol Metab 2006 290(6):E1253-61.10.1152/ajpendo.00572.200416531507 [Google Scholar] [CrossRef] [PubMed]
[5]. Schleinitz D, Genetic determination of serum levels of diabetes-associated adipokinesRev Diabet Stud 2015 12(3-4):277-98.10.1900/RDS.2015.12.27726859657 [Google Scholar] [CrossRef] [PubMed]
[6]. Fain JN, Sacks HS, Buehrer B, Bahouth SW, Garrett E, Wolf RY, Identification of omentin-1 mRNA in human epicardial adipose tissue: comparison to omentin-1 in subcutaneous, internal mammary artery periadventitial and visceral abdominal depotsInt J Obes (Lond) 2008 32(5):810-15.10.1038/sj.ijo.080379018180782 [Google Scholar] [CrossRef] [PubMed]
[7]. Watanabe T, Watanabe-Kominato K, Takahashi Y, Kojima M, Watanabe R, Adipose Tissue-Derived Omentin-1 Function and RegulationCompr Physiol 2017 7(3):765-81.10.1002/cphy.c16004328640441 [Google Scholar] [CrossRef] [PubMed]
[8]. Akour A, Kasabri V, Boulatova N, Bustanji Y, Naffa R, Hyasat D, Levels of metabolic markers in drug-naive prediabetic and type 2 diabetic patientsActa Diabetol 2017 54(2):163-70.10.1007/s00592-016-0926-127752839 [Google Scholar] [CrossRef] [PubMed]
[9]. Moreno-Navarrete JM, Ortega F, Castro A, Sabater M, Ricart W, Fernández-Real JM, Circulating omentin as a novel biomarker of endothelial dysfunctionObesity (silver spring) 2011 19(8):1552-59.10.1038/oby.2010.35121293447 [Google Scholar] [CrossRef] [PubMed]
[10]. Katsi V, Vamvakou G, Lekakis J, Tousoulis D, Stefanadis C, Makris T, Omentin, fat and heart: classical music with new instrumentsHeart, Lung and Circulation 2014 23:802-06.10.1016/j.hlc.2014.03.01324841389 [Google Scholar] [CrossRef] [PubMed]
[11]. Zhong X, Li X, Liu F, Tan H, Shang D, Omentin inhibits TNF-α-induced expression of adhesion molecules in endothelial cells via ERK/NK-κB pathwayBiochem Biophys Res Commun 2012 425(2):401-06.10.1016/j.bbrc.2012.07.11022842465 [Google Scholar] [CrossRef] [PubMed]
[12]. Auguet T, Quintero Y, Riesco D, Terra X, Cresenti A, New adipokines vaspin and omentin. Circulating levels and gene expression in adipose tissue from morbidly obese womenBMC Med Genet 2011 12:6010.1186/1471-2350-12-6021526992 [Google Scholar] [CrossRef] [PubMed]
[13]. Pan HY, Guo L, Li Q, Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetesDiabetes Research and Clinical Practice 2010 88(1):29-33.10.1016/j.diabres.2010.01.01320129687 [Google Scholar] [CrossRef] [PubMed]
[14]. Jialal I, Devaraj S, Kaur H, Adams-Huet B, Bremer AA, Increased chemerin and decreased omentin-1 in both adipose tissue and plasma in nascent metabolic syndromeJ Clin Endocrinol Metab 2013 98(3):E514-17.10.1210/jc.2012-367323303213 [Google Scholar] [CrossRef] [PubMed]
[15]. Tan BK, Adya R, Farhatullah S, Lewandowski KC, O’Hare P, Lehnert H, Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: ex vivo and in vivo regulation of omentin-1 by insulin and glucoseDiabetes 2008 57(4):801-08.10.2337/db07-099018174521 [Google Scholar] [CrossRef] [PubMed]
[16]. Choi JH, Rhee EJ, Kim KH, Woo HY, Lee WY, Sung KC, Plasma omentin-1 levels are reduced in non-obese women with normal glucose tolerance and polycystic ovary syndromeEur J Endocrinol 2011 165(5):789-96.10.1530/EJE-11-037521865408 [Google Scholar] [CrossRef] [PubMed]
[17]. Greulich S, Chen WJ, Maxhera B, Rijzewijk LJ, van der Meer RW, Jonker JT, Cardioprotective properties of omentin-1 in type 2 diabetes: evidence from clinical and in vitro studiesPLoS One 2013 8:e5969710.1371/journal.pone.005969723555749 [Google Scholar] [CrossRef] [PubMed]
[18]. Narumi T, Watanabe T, Kadowaki S, Kinoshita D, Yokoyama M, Honda Y, Impact of serum omentin-1 levels on cardiac prognosis in patients with heart failureCardiovasc Diabetol 2014 13:8410.1186/1475-2840-13-8424755035 [Google Scholar] [CrossRef] [PubMed]
[19]. ZhouHayes JY, Chan L, Zhou SW, Omentin: linking metabolic syndrome and cardiovascular diseaseCurr Vasc Pharmacol 2014 12(5):136-43.10.2174/157016111299914021709503822724476 [Google Scholar] [CrossRef] [PubMed]
[20]. Moreno-Navarrete JM, Catalán V, Ortega F, Gómez-Ambrosi J, Ricart W, Frühbeck G, Circulating omentin concentration increases after weight lossNutrition & Metabolism 2010 7:27-32.10.1186/1743-7075-7-2720380714 [Google Scholar] [CrossRef] [PubMed]
[21]. Lapointe M, Poirier P, Martin J, Bastien M, Auclair A, Cianflone K, Omentin changes following bariatric surgery and predictive links with biomarkers for risk of cardiovascular diseaseCardiovasc Diabetol 2014 13:12410.1186/s12933-014-0124-925139582 [Google Scholar] [CrossRef] [PubMed]
[22]. Urbanová M, Dostálová I, Trachta P, Drápalová J, Kaválková P, Haluzíková D, Serum concentrations and subcutaneous adipose tissue mRNA expression of omentin in morbid obesity and type 2 diabetes mellitus: the effect of very-lowcalorie diet, physical activity and laparoscopic sleeve gastrectomyPhysiol Res 2014 63(2):207-18. [Google Scholar]
[23]. TetracyclineTeratologyWHO Lancet consultation: Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies 2004 363:157-63.10.1016/S0140-6736(03)15268-3 [Google Scholar] [CrossRef]
[24]. Sacks DB, New Delhi. In. Burtis CA, Ashwood ER, Bruns DE (ed). Tietz Fundamentals of Clinical Chemistry 2008 PhiladelphiaSaunders Elsevier:389-91. [Google Scholar]
[25]. Friedwald WT, Levy RI, Fredrickson DS, Estimation of the concentration of low-density lipoprotein cholesterol in Plasma without use of preparative ultracentrifugationClin Chem 1972 18(6):499-502. [Google Scholar]
[26]. Grundy SM, Cleeman JI, Merz CN, Brewer HB, Clark DB, Hunninghake S, Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III GuidelinesJ Am Coll Cardiol 2004 44(3):720-32.10.1016/j.jacc.2004.07.00115358046 [Google Scholar] [CrossRef] [PubMed]
[27]. Goldstein DE, Little RR, Wiedmeyer HM, England JD, Mckenzie EM, Glycated haemoglobin: methodologies and clinical applicationsClin Chem 1986 B32(10 suppl):64-70. [Google Scholar]
[28]. Melmed S, Polonsky K, Larsen PR, Kronenberg H, Williams Textbook of Endocrinology 2011 12th EditionPhiladelphiaElsevier Saunders [Google Scholar]
[29]. Mathews R, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC, Homeostasis model assessment: insulin resistance and beta cell function from fasting glucose and insulin concentration in manDiabetologia 1985 28:412-19.10.1007/BF002808833899825 [Google Scholar] [CrossRef] [PubMed]
[30]. Turner RC, Holman RR, Matthews D, Hockaday TD, Peto J, Insulin deficiency and insulin resistance interaction in diabetes: estimation of their relative contribution by feedback analysis from basal plasma insulin and glucose concentrationsMetabolism 1979 28:1086-96.10.1016/0026-0495(79)90146-X [Google Scholar] [CrossRef]
[31]. Muniyappa R, Lee S, Chen H, Quon MJ, Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usageAm J Physiol Endocrinol and Metab 2008 294(1):E15-26.10.1152/ajpendo.00645.200717957034 [Google Scholar] [CrossRef] [PubMed]
[32]. Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, ICMR-INDIAB collaborative study group. prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian council of medical research-India dIABetes (ICMR-INDIAB) studyDiabetologia 2011 54(12):3022-27.10.1007/s00125-011-2291-521959957 [Google Scholar] [CrossRef] [PubMed]
[33]. Joshi SR, Anjana RM, Deepa M, Pradeepa R, Bhansali A, Dhandania VK, ICMR-INDIAB Collaborative Study Group. Prevalence of dyslipidemia in urban and rural India: the ICMR-INDIAB studyPLoS One 2014 9:e9680810.1371/journal.pone.009680824817067 [Google Scholar] [CrossRef] [PubMed]
[34]. Pradeepa R, Anjana RM, Joshi SR, Bhansali A, Deepa M, Joshi PP, ICMRINDIAB Collaborative Study Group. Prevalence of generalized & abdominal obesity in urban & rural India--the ICMR-INDIAB Study (Phase-I) [ICMRINDIAB- 3]Indian J Med Res 2015 142(2):139-50.10.4103/0971-5916.16423426354211 [Google Scholar] [CrossRef] [PubMed]
[35]. Guo LJ, Jiang TJ, Liao L, Liu H, He HB, Relationship between serum omentin-1 level and bone mineral density in girls with anorexia nervosaJ Endocrinol Invest. 2013 36(3):190-94. [Google Scholar]
[36]. Vu A, Sidhom MS, Bredbeck BC, Kosmiski LA, Aquilante CL, Evaluation of the relationship between circulating omentin-1 concentrations and components of the metabolic syndrome in adults without type 2 diabetes or cardiovascular diseaseDiabetol Metab Syndr 2014 6:410.1186/1758-5996-6-424428913 [Google Scholar] [CrossRef] [PubMed]
[37]. Shibata R, Ouchi N, Takahashi R, Terakura Y, Ohashi K, Ikeda N, Omentin as a novel biomarker of metabolic risk factorsDiabetol Metab Syndr 2012 4(1):3710.1186/1758-5996-4-3722835063 [Google Scholar] [CrossRef] [PubMed]