Introduction
Aluminium and Fluoride are found in abundance on earth. It gets access to our body mainly through drinking water, food stuff, drugs, and utensils. Continuous exposure of aluminium in drinking water can lead to age-associated neurological problems like Alzheimer’s disease and Parkinson’s disease [1]. Similarly, fluoride is also a biologically highly active compound. High level of exposure to it produces oxidative stress, DNA impairment and neuronal damage, which also results in decreased learning and memory ability [2]. When drinking water contains both Fluoride and Aluminium, then there will be the formation of fluoroaluminium complex (AlF3) in the stomach. Its absorption and passage in the bloodstream is more compared to that of its ionic forms and later crosses the Blood Brain Barrier (BBB) [3]. The AlF3 complex produces cell death in the hippocampal CA1 region which is the site of learning and memory when given as 0.1 ppm through drinking water. This also induces abnormal behaviour of the animal, altered neuronal and cerebrovascular integrity [4]. These changes are due to the generation of free radicals, lipid peroxidation and altered antioxidant defense system. Natural products such as flavonoids exert remarkable antioxidant activity which protects from damage occurred due to Aluminium fluoride-induced oxidative stress [5]. The flavonoids normalise the brain damage occurred due to oxidative stress, may be because of its ability to decrease the glutamate-induced increase in intracellular Ca2+ level [6].
Mucuna pruriens (M. pruriens) Linn. which is commonly called as velvet bean or Kapikacchu is one of the widely studied plants for its medicinal effects. It is well-known for its aphrodisiac activities as it is known to increase the sperm count and testosterone levels in the body [7]. It has also been used to treat arthritis, nervous disorder, atherosclerosis, as an antipyretic, analgesic and in Parkinson’s disease [8]. The plant seed contains large amounts of phenolic compounds which exhibit high antioxidant and free radical scavenging activities. As this plant is a natural source of antioxidants, it might be helpful in preventing the oxidative stress. The alcoholic extract shows significant antioxidant activity which was comparable with standard ascorbate and total phenol content [9,10]. However, to date, there are no reports on the effect of Mucuna pruriens against the change in behaviour and neuronal damage which occurred due to AlF3. Taking antioxidant property into the consideration, it can be hypothesised that the extract of Mucuna pruriens might show a protective effect against AlF3 exposure on behavioural and neurological integrity. So, the present study was conducted to assess the possible neuroprotective potential of Mucuna pruriens from the stress occurred due to AlF3 exposure.
Materials and Methods
Preparation of alcoholic plant extract: The Mucuna pruriens plant seeds were powdered and passed through a 40-mesh sieve. First extracted with petroleum ether (-80°C), and then with methanol (95%) for 72 hours. Then this extract was transferred, filtered and lyophilised (-40°C) to attain dry extract which is used for this study.
Animals
The present preclinical experimental study was conducted on 36 male Wistar albino rats each weighing 200 gm-250 gm which were obtained from Central Animal Research Facility, Manipal Academy of Higher Education, Manipal, Karnataka, India. The study was conducted for about four months from January 2016 to April 2016. The rats were accommodated at standard room temperature (23±2°C) in Central Animal House with food and water ad libitum. The protocol of this experiment was accepted by the Institutional Animal Ethics Committee (No. IAEC/KMC/59/2014) and was carried out according to the committee guidelines.
Experimental Design
In present study, we chose six groups with six rats each and allowed to familiarise for seven days prior to the experiment.
Group I-Normal saline, 10 mL/kg, orally.
Group II-Aluminium fluoride (negative control).
Group III-25 mg/kg Quercetin (standard), AlF3 induced stress.
Group IV-50 mg/kg extract, AlF3 induced stress.
Group V-100 mg/kg extract, AlF3 induced stress.
Group VI-200 mg/kg extract, AlF3 induced stress.
Group IV, V and VI rats were fed with extract and Group III rats fed with Quercetin for 10 days. Eleventh day onwards all the rats except Group 1 were treated with AIF33 through the drinking water for seven days in dosage of 600 ppm [6]. After 24 hours, the following tests were conducted:
Spatial learning (T-maze) test: This is a spatial memory task, as described earlier [11] where the ability of the rats to differentiate the left or right arm of the T-maze apparatus to procure food as a reward was observed. In spontaneous alternation test, the mouse was kept in the start box, and allowed to enter into the stem and choose any one of the arms. The arm chosen by the rat in each trial was noted and the percentage bias for each animal was calculated. This was followed by rewarded alternation test which had two parts: forced run and choice run. In the forced run, we closed one arm so that the animal is forced to go to the other arm. In the next step which is a choice run, both arms were made available for the animal. We considered a “correct response” only when a rat enters opposite to the forced arm. Percentage of correct response was calculated
Evaluation of locomotor activity using actophotometer: The locomotor activity in animals was measured using a digital actophotometer (INCO, Ambala, India). Locomotor activity can be an index of wakefulness (alertness) of mental activity. The movement of the animal cuts off a beam of light falling on the photo cell and a count was recorded and displayed digitally. First the animals were weighed and then numbered. Each mouse was placed individually in the actophotometer for about 3 minutes to habituate the animal. Later for next 10 minutes the basal activity score was recorded. The area was cleaned with dilute alcohol and dried between trials to maintain hygienic condition. Decreased activity score was taken as an index of CNS depression [12].
Rotarod test: We conducted this test (Panlab, Harvard apparatus) to assess motor coordination and balance, similar to a previous study [13]. The instrument was adjusted for the speed of 15 rpm and the animal was separately placed on the rotating rod. The latency at which each mouse falls off the rod was documented for a maximum cut-off time for 300 seconds. The procedure was repeated for a total of three trials separated by 15 minutes inter-trial intervals.
Histopathology: After 10 days of behavioural tests the animals were euthanised and the brain was fixed in formalin, embedded and 5 μm thick sections were made, stained with Haematoxylin and Eosin (H&E) for histopathological observation. The hippocampal CA1 region was observed for viable cells which were rounded cells with clear cytoplasm and nuclei. Non viable cells which were shrunken and had fragmented nuclei, were excluded from the count.
Statistical Analysis
The results are represented as mean±SEM with one-way Analysis of variance (ANOVA) and Tukey’s multiple comparisons test as a post-hoc test using a GraphPad Prism version 5.0. The statistical significant value was considered wherever p<0.05.
Results
Spatial Learning (T-maze) Test
Spontaneous alternation tests: During the spontaneous alternation test, animals treated with AlF3 (Group II) showed significant (p<0.05) impairment in spatial learning, in the form of less number of alternations and more percentage bias when compared to the Normal group (Group I). There was an increased number of alternations and decreased percentage bias observed in 50, 100 and 200 mg/kg of extract treated groups (Group IV, V and VI) when compared to that of AlF3 treated group (Group II). A significant difference was observed only in 200 mg/kg of extract treated group (Group VI) and Quercetin treated group (Group III) when compared to AlF3 treated group (Group II) [Table/Fig-1].
Effect of Mucuna pruriens methanolic extract on AlF3 induced behavioral alterations.
| Instrument Used Quercetin+AlF3 | Parameters | Normal | AlF3 | Quercetin+AlF3 | MP (50 mg/kg)+AlF3 | MP (100 mg/kg)+AlF3 | MP (200 mg/kg)+AlF3 |
|---|
| T-maze | Number of Alternations | 14.00±1.00 | 6.67±0.88a | 12.00±1.16b | 9.33±0.67a | 9.67±0.88a | 12.67±0.67b |
| Percentage bias | 51.39±1.39 | 70.83±4.17a | 55.55±1.39b | 63.72±3.52 | 65.11±3.55 | 55.55±3.68b |
| Percentage correct response | 76.22±2.94 | 51.39±1.39a | 67.78±4.94 | 55.55±1.39a | 58.16±6.20a | 74.83±2.55bc |
| Actophotometer Locomotor | 377.00±4.51 | 188.70±2.96a | 363.30±2.33b | 248.00±3.22ab | 330.30±3.38abc | 345.00±3.61abc |
| Rotarod | Motor coordination | 193.30±7.37 | 130.30±6.06a | 176.30±4.33b | 138.30±9.70a | 140.30±5.36a | 179.30±5.49bcd |
Data presented as mean±SEM (n=6), where ap<0.05 compared to normal group, bp<0.05 compared to AlF3 treated group and cp<0.05 compared to MP (50 mg/kg)+AlF3 group and dp<0.05 compared to MP (100 mg/kg)+AlF3 group. p-value calculated by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. For the number of alternations in T-maze the p-value=0.0010 and F (DFn, DFd): F (5,12)=8.953. For the percentage bias in T maze the p-value=0.0077 and F (DFn, DFd): F (5,12)=5.433. For the percentage correct response in T maze the p-value=0.0016 and F (DFn, DFd): F (5,12)=7.994. For the locomotor activity the p<0.0001 and F (DFn, DFd): F (5,12)=476.4. For the motor coordination the p<0.0001 and F (DFn, DFd): F (5,12)=15.93.
Rewarded alternation test: During rewarded alternation test, animals treated with AlF3 (Group II) showed significant (p<0.05) impairment in spatial learning, by decreased percentage correct response in comparison to the Normal group (Group I). A dosedependent increase in percentage correct response was observed in extract treated groups (Group IV, V and VI) as compared to that of AlF3 (Group II) treated group. The increased percentage correct response was also observed in the group treated with Quercetin (Group III) [Table/Fig-1].
Evaluation of locomotor activity using actophotometer: A reduction in the locomotor activity was observed in the animals of Aluminium fluoride-treated group (Group II) in comparison with the animals of the control group (Group I). A dose-dependent significant (p<0.05) increase in locomotor activity was observed in the animals with Mucuna pruriens treated group (Group IV, V and VI) as compared to AlF3 (Group II) treated (negative control) group. Increase in locomotor activity was also observed in Quercetin treated group (Group III) in comparison with that of AlF3 (Group II) treated group [Table/Fig-1].
Rotarod test: A reduction in motor coordination and the balance was seen in the rats of Aluminium fluoride-treated group (Group II) in comparison with that of the control group (Group I). A significant (p<0.05) increase in motor coordination and the balance was observed in the animals with Mucuna pruriens treated group (200 mg/kg) (Group VI) as compared to AlF3 treated (negative control) group (Group II). Increase in motor coordination was also seen in Quercetin treated group (Group III) in comparison with that of AlF3 treated group (Group II) [Table/Fig-1].
Histopathology: Haematoxylin and eosin stained sections of the hippocampal CA1 region showed significantly decreased (p<0.05) number of viable neurons in Aluminium fluoride-treated group (Group II) in comparison with that of the control group (Group I) [Table/Fig-2A,B] Non viable neurons were dark stained, small irregular cells which were distinguished from viable neurons having prominently rounded cells, with clear cell membrane and nuclei. A dose-dependent significant (p<0.05) increase in viability of cells was seen in the animals with Mucuna pruriens treated group (Group IV, V and VI) as compared to AlF3 treated (negative control) group (Group II). Increase in viable cell number was also seen in Quercetin treated group (Group III) in comparison with that of AlF3 treated group (Group II) [Table/Fig-2C-F,3]. Overall, the animals treated with AlF3 showed the neuronal damage. It also reflected the safety of Mucuna pruriens as well as quercetin treated groups.
High power (40X) photomicrographs showing haematoxylin and eosin (Ehrich’s H&E yellowish) stained sections of hippocampal CA1 region. A) Normal saline; B) AlF3; C) Quercetin+AlF3; D-F) 50 mg/kg, 100 mg/kg, and 200 mg/kg of extract respectively+AlF3.
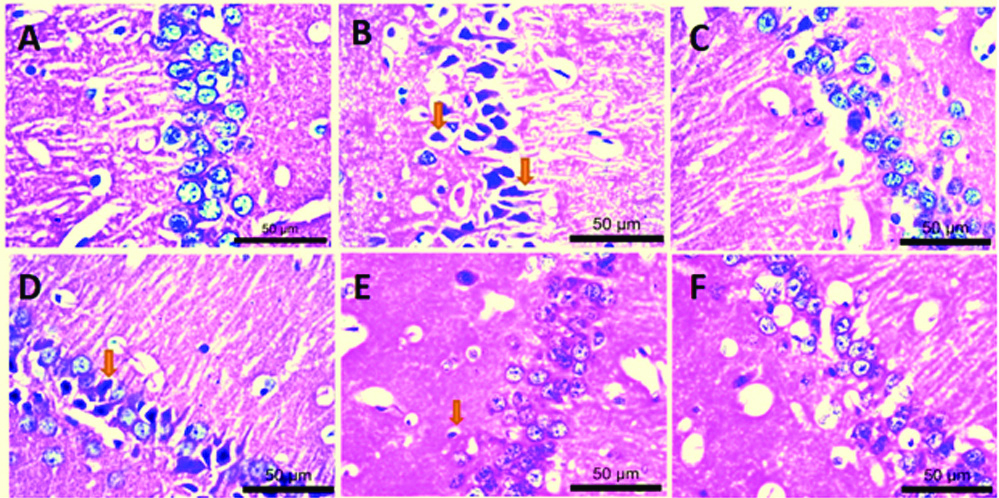
Effect of Mucuna pruriens extract on AlF3 induced histopathological changes.
| Test | Histopathology |
|---|
| Parameters | Viable neurons of CA1 region |
| Normal | 140.0±4.61 |
| AlF3 | 42.00±4.04a |
| Quercetin+AlF3 | 125.3±4.48b |
| MP (50 mg/kg)+AlF3 | 51.67±4.49a |
| MP (100 mg/kg)+AlF3 | 101.7±6.01abc |
| MP (200 mg/kg)+AlF3 | 123.3±3.75bc |
Discussion
It has been reported earlier that chronic aluminium exposure can result in cognitive [14] and locomotor [15,16] impairment. Fluoride anions influence the activity of a variety of enzymes. Fluoroaluminium complex mimics the action of a majority of the neurotransmitters, hormones and growth factors [17]. Fluoride gets collected in the brain and muscle which causes stress and inhibits auto-oxidation mechanism. Thus, it results in oxidative damage of neural and muscular tissue which alters the function of brain and muscle [18]. The present study was conducted to assess the therapeutic potential of previously reported antioxidant principals of methanolic extract of Mucuna pruriens [9] in ameliorating these structural and functional neuromuscular changes induced by AIF33 in Wistar rats.
AIF33 can affect learning and memory [2]. Spatial learning and memory assessment help in evaluating the functional status of neurons. In the present study, the spatial learning ability of the different group of animals was assessed by the T-maze test. Significant learning and memory deficits were found in aluminium fluoride-treated group in the form of less number of alternations, more percentage bias and decreased percentage correct response when compared to the normal group. The treatment with Mucuna pruriens extract showed an increase in spatial learning ability compared to AlF3 control. The reversal of memory deficits by the extract treatment might be due to its neuroprotective effect.
AlF3 affect the cell membrane and integrity of purkinje fiber which results in a decline in motor function [19]. In the present study, we found a similar decline in locomotor activity in AlF3 treated control animals compared to normal control. The reversal in the declined motor activity was observed in Mucuna pruriens extract treated groups when compared with AlF3 treated group. This could be due to intact neuronal integrity which might have prevented a decline in the motor function.
Mucuna pruriens has shown improvement in behavioural profile in various conditions including sexual behavioural profile. These effects indicate alterations in neurotransmitters levels like noradrenaline, dopamine etc., and reports are available for the same [20].
Although, oxygen is necessary for human life, it is also a precursor to the formation of harmful Reactive Oxygen Species (ROS) [21]. The free radicals generated in the body can lead to the formation of ROS which damages the protein, lipid and nucleic acid thus leading to enzyme inactivation, altering the genetic material and cell death [22]. Fluoride is biologically highly active compound and its excessive intake can lead to fluorosis which mainly affects muscles and brain [6]. Studies have also shown that fluoride accumulates in the hippocampal region of rat brain resulting in oxidative stress and neuronal degeneration [2]. Neuronal death may be due to apoptosis or autophagic pathways. This is due to increased glutamate release, the release of ROS, mitochondrial dysfunction or inflammation [23]. In the present study, the hippocampal CA1 region showed a significant decrease (p<0.05) in a number of viable neurons in AIF33 treated group as compared to the animals of the control group.
Natural products with antioxidant properties can be chosen for its maximum therapeutic effect with minimal risk of iatrogenic adverse effects. The neuronal protection is observed in Mucuna pruriens extract treated group which may be due to the antioxidant property of this plant against free radicals. Further studies are required to elucidate the exact mechanism of action.
Limitation
The study could find out behavioural changes only. However, a detailed study for the change in the behavioural parameters at the nuclear level will give a better insight. The functional status of neurons needs to be evaluated using neurotransmitters in the various parts of the brain.
Conclusion
The present study results showed a possible protective role of Mucuna pruriens against AlF3 induced behavioural (such as cognitive deficit and locomotor impairment) and neuronal damage in rat brain. However, the molecular mechanism of the neuroprotective potential of this plant can be assessed by pursuing advanced studies on this plant extract.
Data presented as mean±SEM (n=6), where ap<0.05 compared to normal group, bp<0.05 compared to AlF
3 treated group and cp<0.05 compared to MP (50 mg/kg)+AlF
3 group and dp<0.05 compared to MP (100 mg/kg)+AlF
3 group. p-value calculated by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. For the number of alternations in T-maze the p-value=0.0010 and F (DFn, DFd): F (5,12)=8.953. For the percentage bias in T maze the p-value=0.0077 and F (DFn, DFd): F (5,12)=5.433. For the percentage correct response in T maze the p-value=0.0016 and F (DFn, DFd): F (5,12)=7.994. For the locomotor activity the p<0.0001 and F (DFn, DFd): F (5,12)=476.4. For the motor coordination the p<0.0001 and F (DFn, DFd): F (5,12)=15.93.
[1]. McLachlan DR, Aluminium and the risk for Alzheimer’s diseaseEnvironmetrics 1995 6:233-75.10.1002/env.3170060303 [Google Scholar] [CrossRef]
[2]. Chirumari K, Reddy PK, Dose-dependent effects of fluoride on neurochemical milieu in the hippocampus and neocortex of rat brainFluoride 2007 40:101-10. [Google Scholar]
[3]. Varner JA, Huie CW, Horvath W, Jensen KF, Chronic RL, Chronic AlF3 administration: II. Selected histological observationsNeurosci Res Commun 1993 13:99-104. [Google Scholar]
[4]. Varner JA, Jensen KF, Horvath W, Isaacson RL, Chronic administration of aluminum-fluoride or sodium-fluoride to rats in drinking water: Alterations in neuronal and cerebrovascular integrityBrain Res 1998 784:284-98.10.1016/S0006-8993(97)01336-X [Google Scholar] [CrossRef]
[5]. Ganapaty S, Chandrashekhar VM, Lakshmi Narasu M, Raghavendra HL, Antioxidant activity of natural products against aluminium fluoride induced oxidative stressSci Technol Arts Res J 2012 1(1):26-37.10.4314/star.v1i1.98769 [Google Scholar] [CrossRef]
[6]. Ranpariya VL, Parmar SK, Sheth NR, Chandrashekhar VM, Neuroprotective activity of Matricaria recutita against fluoride-induced stress in ratsPharm Biol 2011 49:696-701.10.3109/13880209.2010.54024921599496 [Google Scholar] [CrossRef] [PubMed]
[7]. Amin KMY, Khan MN, Zillur-Rehman S, Khan S, Sexual function improving effect of Mucuna pruriens in sexually normal male ratsFitoterapia Milano 1996 67:53-56. [Google Scholar]
[8]. Bhaskar A, Nithya V, Vidhya VG, Phytochemical evaluation by GC-MS and antihyperglycemic activity of Mucuna pruriens on Streptozotocin induced diabetes in ratsJ Chem Pharm Res 2011 3:689-96. [Google Scholar]
[9]. Kumar DS, Muthu AK, Smith AA, Manavalan R, In vitro antioxidant activity of various extracts of whole plant of Mucuna pruriens (Linn)Int J Pharm Tech Res 2010 2:2063-70. [Google Scholar]
[10]. Duke AT, Handbook of Medicinal Herbs 1995 3rd EditionLondonCRS Press:220 [Google Scholar]
[11]. Dunnet SB, Low WC, Iverseni SD, Stenvi U, Bjorklund A, Septal transplants restore maze learning in rats with fornix fimbria lesionsBrain Res 1982 251:335-48.10.1016/0006-8993(82)90751-X [Google Scholar] [CrossRef]
[12]. Dews PB, The measurement of the influence of drugs on voluntary activity in miceBr J Pharmacol Chemother 1953 8:46-48.10.1111/j.1476-5381.1953.tb00749.x13066693 [Google Scholar] [CrossRef] [PubMed]
[13]. Uz T, Dimitrijevic N, Tueting P, Manev H, 5-lipoxygenase (5LOX)-deficient mice express reduced anxiety-like behaviourRestor Neurol Neurosci 2002 20:15-20. [Google Scholar]
[14]. Abdel-Aal RA, Assi AA, Kostandy BB, Rivastigmine reverses aluminum-induced behavioural changes in ratsEur J Pharmacol 2011 659:169-76.10.1016/j.ejphar.2011.03.01121440537 [Google Scholar] [CrossRef] [PubMed]
[15]. Erazi H, Sansar W, Ahboucha S, Gamrani H, Aluminum affects glial system and behaviour of ratsC R Biol 2010 333:23-27.10.1016/j.crvi.2009.09.01620176332 [Google Scholar] [CrossRef] [PubMed]
[16]. Nampoothiri M, John J, Kumar N, Mudgal J, Nampurath GK, Chamallamudi MR, Modulatory role of simvastatin against aluminium chloride-induced behavioural and biochemical changes in ratsBehav Neurol 2015 2015:21016910.1155/2015/21016925802481 [Google Scholar] [CrossRef] [PubMed]
[17]. Sternweis PC, Gilman AG, Aluminium: a requirement for activation of the regulatory component of adenylate cyclase by fluorideProc Natl Acad Sci USA 1982 79(16):4888-91.10.1073/pnas.79.16.48886289322 [Google Scholar] [CrossRef] [PubMed]
[18]. Vani ML, Reddy KP, Effects of fluoride accumulation on some enzymes of brain and gastrocnemius muscle of miceFluoride 2000 33:17-26. [Google Scholar]
[19]. Kaur T, Bijarnia RK, Nehru B, Effect of concurrent chronic exposure of fluoride and aluminum on rat brainDrug and Chemical Toxicology 2009 32(3):215-21.10.1080/0148054090286225119538017 [Google Scholar] [CrossRef] [PubMed]
[20]. Suresh S, Prakash S, Effect of Mucuna pruriens (Linn.) on oxidative stressinduced structural alteration of corpus cavernosum in streptozotocin-induced diabetic ratJ Sex Med 2011 8:1943-56.10.1111/j.1743-6109.2011.02221.x21366877 [Google Scholar] [CrossRef] [PubMed]
[21]. Albert YS, Yong-Mei C, Oxidative stress and neurodegenerative disordersJ Biomed Sci 1998 5:401-14.10.1007/BF022559289845843 [Google Scholar] [CrossRef] [PubMed]
[22]. Halliwell B, Reactive oxygen species in living systems: source, biochemistry, and role in human diseaseAm J Med 1991 91:14S-22.10.1016/0002-9343(91)90279-7 [Google Scholar] [CrossRef]
[23]. Nikonenko AG, Radenovic L, Andjus PR, Skibo GG, Structural features of ischemic damage in the hippocampusAnat Rec (Hoboken) 2009 292:1914-21.10.1002/ar.2096919943345 [Google Scholar] [CrossRef] [PubMed]