Introduction
Breast cancer is the most frequent cancer among women with an estimated 1.67 million new cases diagnosed in 2012 [1]. It has ranked number one in Indian women with age adjusted rate being as high as 25.8 per 100,000 women [2]. Breast cancer has surpassed cervical cancer and is now the leading cancer of cancer death, however cervical cancer still remains common in rural areas [3].
Broadly, breast carcinomas can be divided into invasive and in situ carcinoma. The incidence of DCIS of the breast seems to be increasing markedly, particularly with the advent of mammography [4]. DCIS is conventionally regarded as a precursor of invasive breast carcinoma [5] and it has long been recognised that in some patients, DCIS will progress to invasive carcinoma if it is untreated or treated inadequately. When the disease is recurrent, almost half of the cases show invasive histologic features. The progression of DCIS to IDC may be from low grade DCIS to low grade IDC and high grade DCIS to high grade IDC. This finding is supported by the parallel or horizontal model [6] of breast cancer progression.
Studies comparing the immunohistochemical and genetic characteristics of DCIS with those of invasive carcinomas have identified similar changes between low grade DCIS and low grade invasive cancers, and similarly between high grade DCIS and high grade invasive cancers. Comparative genomic hybridisation studies of chromosome deletion demonstrated that 65% of low grade tumours had lost the long arm of chromosome 16 compared with only 16% of high grade tumours. This model implies ‘‘commitment’’ of a subtype of DCIS to a specific subtype of invasive carcinoma (high grade or low grade): low grade DCIS tends to progress to low grade, while high grade DCIS tends to progress to high grade invasive carcinoma. Besides studies of chromosomal alterations, this model is also supported by histologic data showing that in the progression of DCIS to invasive disease, the grade of DCIS consistently corresponds to the grade of subsequent invasive carcinoma [7].
This concept differs from the conventional model of disease progression in vulva, vagina, cervix and skin where there is increasing grades of dysplasia before development of invasive carcinoma [2].
In view of this, the present study was undertaken to analyse the relation between the grades of concurrent DCIS and IDC and to correlate these with the expression of biological markers like ER, PR and HER-2/neu.
This study aims at correlation of the histological grades of concurrent in situ and invasive carcinomas and their immunohistochemical parameters. The study was undertaken to highlight the natural history of progression of breast DCIS to IDC.
Materials and Methods
Present study was a prospective study carried out at the Department of Pathology, Father Muller Medical College, Mangaluru for a period of two years and 3 months starting from January 2011 to February 2013. A total number of 60 mastectomy/lumpectomy cases were included in the study. Specimens that showed concurrent IDC (NOS) and DCIS were included in the study. Specimens that did not contain DCIS, those with lobular carcinoma or any other invasive carcinomas were excluded. Sections from formalin fixed, paraffin embedded tissue were stained with H&E stain.
IDC was graded according to the Nottingham modification of the Bloom and Richardson system [8]. DCIS was graded according to the Lagios classification [4] [Table/Fig-1] which evaluated nuclear grade, architecture and necrosis.
Lagios classification [4] of grading DCIS.
Notes: pes of implan.
| Features evaluated | Low grade | Intermediate grade | High grade |
|---|
| Nuclear diameter | 1-1.5 times of RBC | 1-2 times of RBCs | >2 times of RBCs |
| Chromatin | Diffuse | Coarse | Coarse |
| Nucleoli | Absent | Infrequent | 1 or more nucleoli |
| Mitoses | <1 per 10 HPF | 1-2 per 10 HPF | >2 per 10 HPF |
| Necrosis | Absent | 1+ | 2+ |
Representative blocks from all tumours were subjected to immunostaining for three markers, ER, PR and HER-2/neu. All primary antibodies were used following pressure cooker stimulated antigen retrieval method. The antibodies used were from Biogenex company with the antibody clones being 1D5, PR88 and EP1045Y for anti estrogen receptor, anti progesterone receptor and anti HER-2 respectively. Immunostaining in DCIS and IDC components were evaluated independently. ER and PR scores were given as mild, moderate and strongly positive based on H-score awarded [9]. HER-2/neu was scored as negative, 1+, 2+, 3+ based on the intensity of membranous staining.
Statistical Analysis
All the data were entered into Microsoft Excel 2010 spreadsheet and analysed using SSPS software version 13.0. Descriptive statistics were derived using frequency, percentage, mean, standard deviation. Chi-square test and Fisher’s exact-test were used to test the significance i.e., nominal significance at p-value ≤0.05 level, for high significance the p-value was ≤0.01 and for not significant the p-value was >0.05.
Results
The age of the patients ranged from 29 to 82 years in present study, with mean age of 48.6 years (SD±11.21). Left breast upper outer quadrant was the most common anatomical location (57%). The mean tumour size was 3.55 cms (SD±1.45). We found that most common grade of IDC was Grade 2, seen in 35 patients (58.3%) followed by Grade 3 in 23 patients (38.8%) and Grade 1 in 2 patients (3.3%), whereas high grade DCIS was predominant i.e., in 38 patients (63.3%) followed by intermediate grade in 19 patients (31.7%). One patient (1.7%) had intermediate to high grade DCIS whereas 2 (3.3%) showed a combination of low and high grade DCIS. The grade of the IDC and coexisting in situ carcinoma was found to be concordant in 40 (66.66%) cases. Of these, 18 (30%) cases had intermediate grade [Table/Fig-2] and 22 (36.66%) showed high grade features in both IDC and DCIS components. In the remaining discordant cases, 16 (26.66%) showed the IDC component to be Grade 2 whereas DCIS was high grade [Table/Fig-3]. In one case of each Grade 1 and Grade 2 IDC (i.e., a total of 3.66%), the DCIS component exhibited both low grade and high grade features [Table/Fig-2]. Likewise, one case (1.66%) of Grade 3 IDC had coexisting intermediate to high grade DCIS component. And one case (1.66%) of Grade 1 IDC showed intermediate grade DCIS. The various grades of IDC and DCIS are depicted in [Table/Fig-4]. The statistical correlation between the two showed high significance (p<0.001, HS).
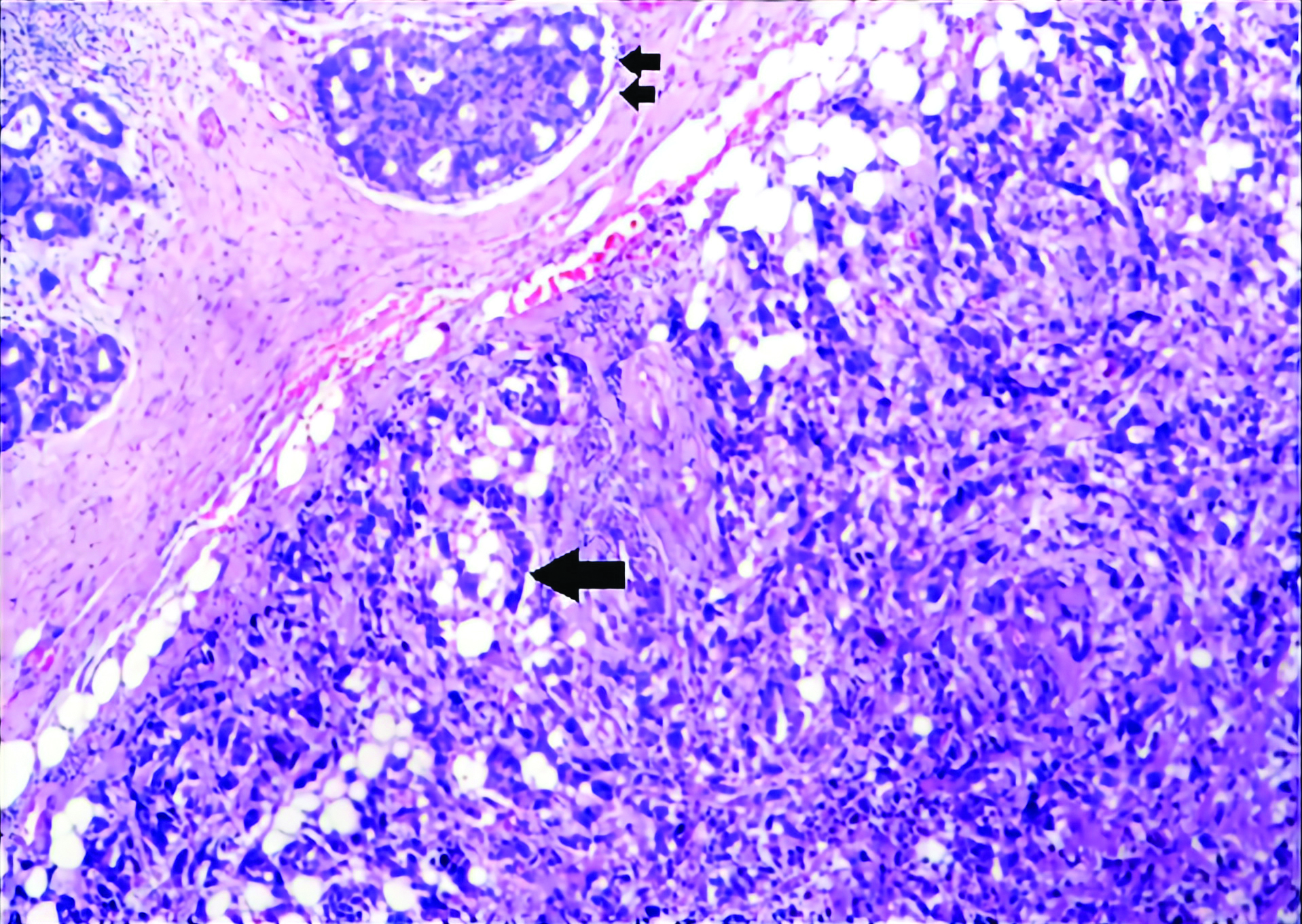
Concurrent infiltrating duct carcinoma (single arrow) and cribriform intraductal carcinoma (double arrow) exhibiting same nuclear grade (Grade 2) (H&E stain; 10X).
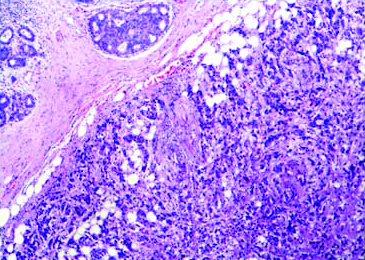
Discordant morphology-Grade 2 infiltrating duct carcinoma with high grade DCIS (H&E Stain; 10X) Inset shows DCIS showing highly pleomorphic cells with prominent nucleoli (H&E stain; 40X).
Correlation between IDC and DCIS grades.
| IDC grades | High Grade DCIS | Intermediate Grade DCIS | Intermediate+High Grade DCIS | Low grade DCIS | Total |
|---|
| IDC Grade 1 | 0 | 1 | 1 | 0 | 2% |
| IDC Grade 2 | 16 | 18 | 1 | 0 | 35% |
| IDC Grade 3 | 22 | 0 | 1 | 0 | 23% |
| | | | | 60% |
Fishe’s exact-test p<0.001, HS
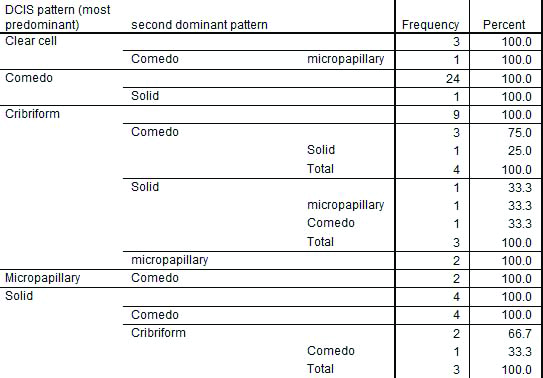
The most predominant pattern of DCIS was comedo (41.7%) followed by cribriform (30%). Other patterns included solid (18.3%), clear cell (6.7%) and micropapillary (3.3%). Various DCIS patterns are depicted in [Table/Fig-5].
Table depicting various DCIS patterns
A correlation between IDC grades and patterns of DCIS was also attempted which was statistically not found to be significant (Fisher’s exact test p=0.203, NS) [Table/Fig-6].
Correlation between IDC grades and patterns of DCIS
| IDC grades | Clear cell DCIS | Comedo DCIS | Cribriform DCIS | Micropapillary DCIS | Solid DCIS | Total |
|---|
| IDC Grade 1 | 0 | 0 | 2 | 0 | 0 | 2 |
| IDC Grade 2 | 3 | 11 | 12 | 1 | 8 | 35 |
| IDC Grade 3 | 1 | 14 | 4 | 1 | 3 | 23 |
| Total | 4 | 25 | 18 | 2 | 11 | 60 |
Fisher’s exact-test p=0.203, NS
A total number of 50 cases (83.33%) showed negative ER in both IDC and DCIS components. In the remaining, 3 (5%) were strong positive, 1 case (1.66%) showed moderate and 5 (8.33%) showed weak positivity in both components. Variable expression was seen in 1 case (1.66%) with DCIS component being ER positive and IDC exhibiting weak positivity. Statistical correlation was significant (Fisher’s exact-test p=0.034, significant).
A total of 47 cases were found to be PR negative and 11 were PR positive in both DCIS and IDC components. Two cases of strong positive PR in IDC component showed variable expression with DCIS being PR negative in one case and weakly positive in the other case. Overall, the PR expression in IDC and concurrent DCIS components was statistically significant (Fisher’s exact test p=0.001, HS).
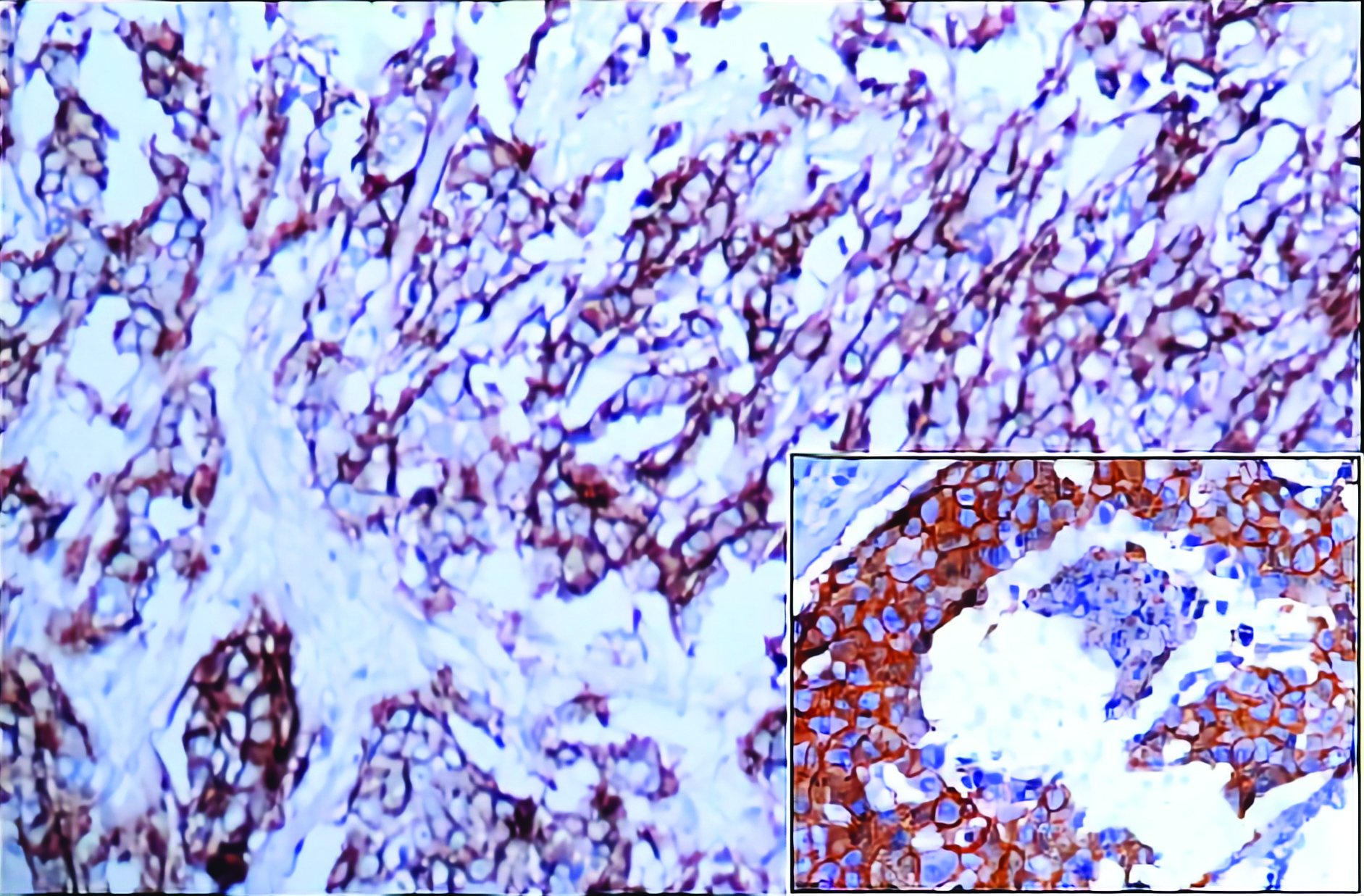
In this study, 98.33% cases showed similar HER-2/neu expression in both IDC and DCIS components [Table/Fig-7]. HER-2/neu was over expressed in 10 (16.66%) cases in which 1 (1.66%) case showed HER-2/neu negativity (1+) in IDC component and 2+ in DCIS. Rest of the cases were HER-2/neu negative (p<0.001, HS).
Invasive duct carcinoma, HER-2/neu : Membrane immunoreactivity (3+) (40X). Inset shows concurrent comedo type DCIS exhibiting HER-2/neu positivity (3+) (40X).
Discussion
In India the incidence of breast cancer is at a constant rise. Many studies have been published studying the biological behaviour of the disease. The average age of the patient at the time of presentation is 45-50 years as observed by Putti TH et al., [10], Mudduwa LKB, [11], Ahmed HG et al., [12], and Gupta S et al., [13] in their studies. Similar results were observed in present study with the mean age of patients being 48.6 years. The mean tumour size was 3.55 cms which was comparable to results obtained by Mudduwa LKB, [11] and Sofi GN et al., [14].
In present study, Grade 2 IDC was most predominant grade observed as also seen by Bhagat VM et al., [15], Ahmed HG et al., [12], Pathak TB et al., [16], and Sofi GN et al., [14]. However, Cadman BA et al., [17] and Mudduwa LKB, [12] in their studies found Grade 3 IDC as predominant grade.
In this study, we used Lagios classification to grade DCIS and found high grade DCIS in 63.3% cases. Our results were found to be concordant with Cadman BA et al., [17], whereas deviation in results were seen between our study and studies by Fisher ER et al., [18] and Latta EK et al., [19], where intermediate grade DCIS was predominant. In the present study, we employed Lagios classification [4] for grading DCIS as there are two major drawbacks in the application of systems like Bellamy COC et al., [20] and Hollande R et al., [21] that use a combination of nuclear grade and architectural pattern. One is the heterogeneity of architectural patterns within the same lesion; the other is the variation of nuclear grade within the same pattern subtype. For example, cribriform DCIS can show low, intermediate, and, less commonly, high nuclear grades. This problem can be avoided to a certain extent by using the Lagios classification and Van Nuys system which uses nuclear grade as the main criterion with further subdivision by the presence or absence of necrosis [4].
In the present study, the grade of the IDC and coexisting in situ carcinoma was found to be concordant in 40 (66.66%) cases. In comparison, the study by Gupta S et al., [13] shows the nuclear grade of the DCIS and coexisting invasive carcinoma to be concordant in 94.67% cases. In the remaining 5.33% cases in the above mentioned study, DCIS demonstrated a high nuclear grade while the invasive component was low nuclear grade as also seen in our study, where 16 (26.66%) cases showed the IDC component to be intermediate grade whereas DCIS was high grade.
The ER and PR receptor expression was 16.7% and 21.7% respectively in present study. The experience of other studies i.e., Bhagat VM et al., [15] Shet T et al., [22] Nisa A et al., [23] and Mudduwa LKB, [12] were not same as present study experiences. In most of these studies a higher proportion of tumours were ER and PR positive. ER and PR coexpression in our study were as follows ER+/PR+(11.6%), ER+/PR- (5%), ER-/PR+ (10%) and ER-/ PR-(73.33%). As against the above quoted studies, our study showed a deviation in the results of ER and PR coexpression.
The percentage of HER-2/neu positive tumours in our study was 16.6 % which was comparable with results of Haung HJ et al., [24]. Bhagat VM et al., [15] and Lal P et al., [25] encountered a higher percentage of HER-2/neu positive tumours in their studies
Till date only few studies have been attempted to evaluate whether the same molecular subtypes as identified in invasive cancer, are also seen in DCIS. In present study, 50/60 (83.33%) cases showed negative ER in both IDC and DCIS components, 3 (5%) were strong positive, 1 (1.66%) case showed moderate and 5 (8.33%) showed weak positivity in both components. Variable expression was seen in 1 (1.66%) case with DCIS component being ER positive and IDC exhibiting weak positivity. Overall, 98.33% of cases showed similar expression of ER, PR and HER-2/neu in both the components.
In this study, we found concordant morphological grades of IDC and DCIS and expression of ER, PR and HER-2/neu by these components. This proves the fact that DCIS unlike other epithelial malignancies like carcinoma cervix and carcinoma vulva follow a parallel or horizontal progression to invasive carcinomas rather than a vertical progression.
Limitation
Non inclusion of Ki-67 is a limiting factor of this study as Ki-67 labelling plays utmost role in assessing disease behavior
Conclusion
The incidence of breast cancer in India is increasing, hence appropriate screening, diagnostic and therapeutic modalities are need of the hour. A better understanding of the nature of breast cancer with respect to progression from in situ to invasive carcinoma can help in planning appropriate treatment and also predict the prognosis, thereby helping in better patient care, as more aggressive treatment could be given to women with high grade DCIS lesions which are at highest risk of progressing to invasive cancer.
Fishe’s exact-test p<0.001, HS
Fisher’s exact-test p=0.203, NS
[1]. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J cancer 2015 136:E359-86.10.1002/ijc.2921025220842 [Google Scholar] [CrossRef] [PubMed]
[2]. Gupta A, Shridhar K, Dhillon PK, A review of breast cancer awareness among women in India: cancer literate or awareness deficit?Eur J Cancer 2015 51:2058-66.10.1016/j.ejca.2015.07.00826232859 [Google Scholar] [CrossRef] [PubMed]
[3]. Kaarthigeyan K, Cervical cancer in India and HPV vaccinationIndian J Med Paediatr Oncol 2012 33:07-12.10.4103/0971-5851.9696122754202 [Google Scholar] [CrossRef] [PubMed]
[4]. Leong AY, Sormunen RT, Vinyuvat S, Hamdani RW, Biologic markers in ductal carcinoma in situ and concurrent infiltrating carcinomaAm J Clin Pathol 2001 115:709-16.10.1309/WBU9-22QN-C3NA-2Q1211345835 [Google Scholar] [CrossRef] [PubMed]
[5]. Pinder SE, Ellis IO, Atypical ductal hyperplasia, ductal carcinoma in situ and in situ atypical apocrine proliferations of the breastCancer Diagnostic Pathol 1996 3:235-42.10.1016/S0968-6053(96)80005-6 [Google Scholar] [CrossRef]
[6]. Klein CA, Parallel progression of primary tumours and metastasesNat Rev Cancer 2009 9:302-12.10.1038/nrc262719308069 [Google Scholar] [CrossRef] [PubMed]
[7]. Acs G, Ductal carcinoma in situ : current conceptsPatología 2010 48(3):180-93. [Google Scholar]
[8]. Elston CW, Ellis IO, Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: experience from a large study with long-term follow-upHistopathology 1991 19:403-10.10.1111/j.1365-2559.1991.tb00229.x1757079 [Google Scholar] [CrossRef] [PubMed]
[9]. Collins LC, Botero ML, Schnitt SJ, Bimodal frequency distribution of estrogen receptor immunohistochemical staining results in breast cancerAm J Clin Pathol 2005 123:16-20.10.1309/HCF035N9WK40ETJ015762275 [Google Scholar] [CrossRef] [PubMed]
[10]. Putti TH, El-Rehim DM, Rakha EA, Paish CE, Lee AHS, Pinder SE, Estrogen receptor-negative breast carcinomas: a review of morphology and immunophenotypical analysisMod Pathol 2005 18:26-35.10.1038/modpathol.380025515332092 [Google Scholar] [CrossRef] [PubMed]
[11]. Mudduwa LKB, Quick score of hormone receptor status of breast carcinoma: correlation with other clinicopathological prognostic parametersIndian J Pathol Microbiol 2009 52(2):159-63.10.4103/0377-4929.4890619332901 [Google Scholar] [CrossRef] [PubMed]
[12]. Ahmed HG, Al-Adhraei MA, Al-Thobhani AK, Correlations of hormone receptors (ER and PR), HER-2/neu and p53 expression in breast ductal carcinoma among Yemeni womenOpen Cancer Immunology J 2011 4:01-09.10.2174/1876401001104010001 [Google Scholar] [CrossRef]
[13]. Gupta S, Deka L, Gupta R, Pant L, Singh S, Molecular phenotypes of ductal carcinoma in situ and invasive ductal carcinoma: a comparative studyIndian J Pathol Microbiol 2012 55(1):43-46.10.4103/0377-4929.9485422499299 [Google Scholar] [CrossRef] [PubMed]
[14]. Sofi GN, Sofi JN, Nadeem R, Shiekh RY, Khan FA, Sofi AA, Estrogen receptor and progesterone receptor status in breast cancer in relation to age, histological grade, size of lesion and lymph node involvementAsian Pacific J Cancer Prev 2012 13(10):5047-52.10.7314/APJCP.2012.13.10.504723244108 [Google Scholar] [CrossRef] [PubMed]
[15]. Bhagat VM, Jha BM, Patel PR, Correlation of hormonal receptor and HER-2/neu expression in breast cancer: a study at tertiary care hospital in south GujaratNational J Med Res 2012 2(3):295-98. [Google Scholar]
[16]. Pathak TB, Bashyal R, Pun CB, Shrestha S, Bastola S, Neupane S, Estrogen and progesterone receptor expression in breast carcinomaJournal of Pathology of Nepal 2011 1(2):100-03.10.3126/jpn.v1i2.5401 [Google Scholar] [CrossRef]
[17]. Cadman BA, Ostrowski JL, Quinn CM, Invasive ductal carcinoma accompanied by ductal carcinoma in situ (DCIS): Comparison of DCIS grade with grade of invasive componentBreast 1997 16:132-37.10.1016/S0960-9776(97)90553-1 [Google Scholar] [CrossRef]
[18]. Fisher ER, Sass R, Fisher B, Wickerham L, Paik SL, Pathologic findings from the national surgical adjuvant breast project-lntraductal carcinoma (dcis)Cancer 1986 57:197-208.10.1002/1097-0142(19860115)57:2<197::AID-CNCR2820570203>3.0.CO;2-N [Google Scholar] [CrossRef]
[19]. Latta EK, Tjan S, Parkes RK, O’Malley FP, The Role of HER2/neu overexpression/ amplification in the progression of ductal carcinoma in situ to invasive carcinoma of the breastMod Pathol 2002 15(12):1318-25.10.1097/01.MP.0000038462.62634.B112481013 [Google Scholar] [CrossRef] [PubMed]
[20]. Bellamy COC, Mc Donald C, Salter DM, Chetty U, Anderson TJ, Non invasive ductal carcinoma of breast: relevance of histologic categorizationHum Pathol 1993 24:16-23.10.1016/0046-8177(93)90057-N [Google Scholar] [CrossRef]
[21]. Holland R, Peterse JL, Millis RR, Eusebi V, Faverly D, van de Vijver MJ, Ductal carcinoma in situ: a proposal for a new classificationSemin Diaggn Pathol 1994 11:167-88. [Google Scholar]
[22]. Shet T, Agrawal A, Nandkarni M, Palkar M, Havaldar R, Parmar V, Hormone receptors over the last 8 years in a cancer referral centre in India: what was and what is ?Indian J Pathol Microbiol 2009 52(2):171-74.10.4103/0377-4929.4890919332904 [Google Scholar] [CrossRef] [PubMed]
[23]. Nisa A, Bhurgri Y, Raza F, Kayani N, Comparison of ER, PR & HER-2/neu (C-erb B 2) reactivity pattern with histologic grade, tumour size and lymph node status in breast cancerAsian Pacific J Cancer Prev 2008 9:553-56. [Google Scholar]
[24]. Huang HJ, Neven P, Drijkoningen M, Paridaens R, Wildiers H, Van Limbergen E, Association between tumour characteristics and HER-2/neu by immunohistochemistry in 1362 women with primary operable breast cancerJ Clin Pathol 2005 58:611-16.10.1136/jcp.2004.02277215917412 [Google Scholar] [CrossRef] [PubMed]
[25]. Lal P, Tan LK, Chen B, Correlation of HER-2 Status With Estrogen And Progesterone Receptors And Histologic Features In Invasive Breast CarcinomasAm J Clin Pathol 2005 123:541-46.10.1309/YMJ3A83TB39MRUT915743737 [Google Scholar] [CrossRef] [PubMed]