Rituximab Therapy in Pulmonary Alveolar Proteinosis: A Rare Case Report
Fatemeh Aghaei Meybodi1, Salar Khazani Fard2, Mojtaba Babaei Zarch3, Mostafa Babai4
1 Assistant Professor, Department of Internal Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2 Resident, Department of Internal Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3 Medical Student, Student Research Committee, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4 Resident, Department of Radiology, Ahvaz Jundishapour University of Medical Sciences, Ahvaz, Iran.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Mr. Mojtaba Babaei Zarch, Shahid Sadoughi Hospital, Ebne-sina Street, Yazd, Iran.
E-mail: mojtaba.babaei72@yahoo.com
Pulmonary Alveolar Proteinosis (PAP) is a rare disorder characterised by accumulation of surfactant in alveoli due to impaired surfactant clearance. Although, whole lung lavage is the standard treatment of PAP, rituximab has also been introduced as a therapeutic option for PAP. A 49-year-old female patient, a known case of PAP, came to the outpatient clinic complaining of exacerbation of dyspnoea. The patient was treated with rituximab four times in a year. Finally, the clinical status and spirometry tests showed improvement. Although, the main treatment for PAP is whole lung lavage, other therapeutic options may be useful too. According to the prior case reports and the present one, rituximab can be useful in treatment of PAP.
Anti-CD20 monoclonal antibody, Ground glass opacity, Surfactant
Case Report
A 49-year-old female, a housewife and a known case of PAP reported with exacerbation of dyspnoea. She had initial symptoms (dyspnoea during exercise, cough, and sputum) of PAP, which persisted for four years. She had history of passive smoking with no orthopnoea, Paroxysmal Nocturnal Dyspnoea (PND), bloody sputum, and history of allergy. Vital signs included: respiratory rate-26/minute; pulse rate-84 beats/minute; temperature-98.4°F; blood pressure-110/70 mmHg, and oxygen saturation of 93% on room air.
There was no evidence of cervical lymphadenopathy. Heart sounds were normal. The lungs were clear on auscultation. There was no clubbing, cyanosis or oedema in the limbs. The patient was hospitalised. Spirometry showed a mild-to-moderate restrictive pattern [Table/Fig-1].
| Flow/Volume | Before initiating rituximab | 1 month later | 7 months later | 1 year later |
|---|
| FVC |
| Pre/Pep | 63% | 60% | 59% | 72% |
| Post/Pred | 66% | 65% | 60% | 68% |
| FEV1 |
| Pre/Pep | 56% | 57% | 59% | 69% |
| Post/Pred | 65% | 62% | 60% | 63% |
| Difference | 17% | 9% | 1% | -9% |
| FEV1/FVC |
| Pre/Pep | 94% | 101% | 107% | 102% |
| Post/Pred | 106% | 101% | 107% | 99% |
| MEF25-75 |
| Pre/Pep | 36% | 42% | 50% | 48% |
| Post/Pred | 32% | 42% | 53% | 42% |
| Oxygen saturation | 93% | 92% | 93% | 95% |
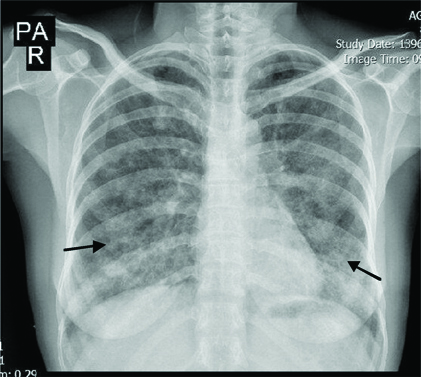
Chest X-ray revealed ground glass opacities in the upper zone and patchy opacity in the lower zones of both lungs [Table/Fig-2].
Chest X-ray showing ground glass opacities in both lungs.
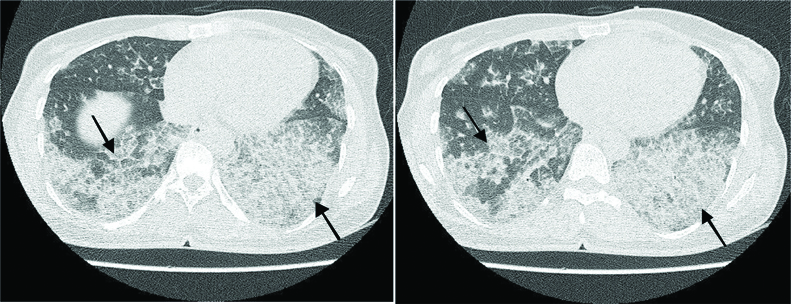
Laboratory tests were normal except hypochromic microcytic anaemia. The patient was treated with rituximab (800 mg rituximab in normal saline). In addition, acetaminophen, chlorpheniramine, and hydrocortisone were injected before administration of rituximab. Finally, the patient was discharged with a good condition after four days. After one month, she was again referred for increased dyspnoea and was treated again with rituximab (800 mg). High-Resolution Computed Tomography (HRCT) showed ground glass opacities with geographic pattern and interlobular septal thickening (crazy paving pattern), specifically in the left-side lung, indicating the occurrence of PAP [Table/Fig-3]. The patient received rituximab two more times (7 and 12 months after the first admission). The last spirometry (one-year after initiating rituximab) showed a mild restrictive pattern, indicating an acceptable improvement compared to the mild-to-moderate pattern in the past year.
HRCT showing crazy paving pattern. It is characterised as ground glass opacities distributed in lung parenchyma, along with interlobular and interlobar septal thickening.
Discussion
Pulmonary alveolar proteinosis is a rare disorder caused by accumulation of surfactant in alveoli due to impaired surfactant clearance, resulting in respiratory problems [1,2]. Its annual prevalence is 0.36-0.49 per one million people. PAP has three aetiologically distinct forms, namely autoimmune {Anti-granulocyte-macrophage Colony Stimulating Factor antibodies (anti-GM-CSF antibodies)}, genetic (mutation of surfactant proteins or GM-CSF receptor gene), and secondary (inhalation of toxic substances or haematologic diseases). The most common form of PAP is autoimmune, accounting for 90% of all PAP cases [3-5].
Clinical symptoms of PAP include dyspnoea during exercise, cough, fatigue, and weight loss. Clinical examination is often non specific, but cyanosis, clubbing, and crackling have been reported in some patients [6]. Initial symptoms of our patient were dyspnoea during exercise and cough. There was no evidence of cyanosis or clubbing. The lung auscultation was normal. PAP diagnosis is usually done using CT scan and staining of lung lavage fluid [1]. HRCT showed the crazy paving pattern in the patient, characterised by ground glass opacities distributed in lung parenchyma, along with interlobular and interlobar septal thickening [7]. The clinical course of PAP varies from spontaneous recovery to respiratory failure and even death [3]. Spontaneous recovery may occur in 28% of PAP patients [8]. The standard treatment for PAP is symptomatic and whole lung lavage therapies, that has been proven to be effective in more than 60% of all cases; in addition, 10% of patients are resistant to lavage [9,10].
Whole lung lavage is performed under general anaesthesia. Fluid leakage may occur following mechanical ventilation of one lung in a hypoxic patient. Nowadays, other therapeutic options, such as GM-CSF supplementation, plasmapheresis, and rituximab, have been introduced for PAP [11].
Rituximab is an anti-CD20 monoclonal antibody of B-lymphocytes. A study showed that rituximab improves the macrophage lipid transfer, macrophage lipid metabolism, and subsequently surfactant catabolism through reducing anti-GM-CSF autoantibodies [2]. Moreover, previous findings showed that rituximab reduced anti-GM-CSF antibody in bronchoalveolar lavage fluid of PAP patients; however, the serum level was not significantly affected [12]. The present patient received four rituximab therapies in one year, and showed an acceptable improvement in clinical outcome and spirometry test results.
Conclusion
Although, the whole lung lavage is the standard PAP care, results from previous case studies and the present case showed that rituximab can be used as a therapeutic option in patients with PAP.
[1]. Borie R, Danel C, Debray MP, Taille C, Dombret MC, Aubier M, Pulmonary alveolar proteinosisEuropean Respiratory Review 2011 20(120):98-107.10.1183/09059180.0000131121632797 [Google Scholar] [CrossRef] [PubMed]
[2]. Malur A, Kavuru MS, Marshall I, Barna BP, Huizar I, Karnekar R, Rituximab therapy in pulmonary alveolar proteinosis improves alveolar macrophage lipid homeostasisRespiratory Research 2012 13(1):4610.1186/1465-9921-13-4622697800 [Google Scholar] [CrossRef] [PubMed]
[3]. Mo Q, Wang B, Dong N, Bao L, Su X, Li Y, The clinical clues of pulmonary alveolar proteinosis: a report of 11 cases and literature reviewCanadian Respiratory Journal 2016 2016:402192810.1155/2016/402192827445535 [Google Scholar] [CrossRef] [PubMed]
[4]. Delaval P, Brinchault G, Corre R, Jouneau S, Meunier C, Briens E, Pulmonary alveolar phospholipoproteinosisRev Pneumol Clin 2005 61(3):186-92.10.1016/S0761-8417(05)84811-4 [Google Scholar] [CrossRef]
[5]. Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, Hizawa N, Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in JapanAmerican Journal of Respiratory and Critical Care Medicine 2008 177(7):752-62.10.1164/rccm.200708-1271OC18202348 [Google Scholar] [CrossRef] [PubMed]
[6]. Suzuki T, Trapnell BC, Pulmonary alveolar proteinosis syndromeClinics in Chest Medicine 2016 37(3):431-40.10.1016/j.ccm.2016.04.00627514590 [Google Scholar] [CrossRef] [PubMed]
[7]. Rossi SE, Erasmus JJ, Volpacchio M, Franquet T, Castiglioni T, McAdams HP, “Crazy-paving” pattern at thin-section CT of the lungs: radiologic-pathologic overviewRadiographics 2003 23(6):1509-19.10.1148/rg.23603510114615561 [Google Scholar] [CrossRef] [PubMed]
[8]. Borie R, Debray MP, Laine C, Aubier M, Crestani B, Rituximab therapy in autoimmune pulmonary alveolar proteinosisEuropean Respiratory Journal 2009 33(6):1503-06.10.1183/09031936.0016090819483052 [Google Scholar] [CrossRef] [PubMed]
[9]. Papiris SA, Tsirigotis P, Kolilekas L, Papadaki G, Papaioannou AI, Triantafillidou C, Pulmonary alveolar proteinosis: time to shift?Expert Review of Respiratory Medicine 2015 9(3):337-49.10.1586/17476348.2015.103525925864717 [Google Scholar] [CrossRef] [PubMed]
[10]. Ben-Dov I, Segel MJ, Autoimmune pulmonary alveolar proteinosis: clinical course and diagnostic criteriaAutoimmunity Reviews 2014 13(4):513-17.10.1016/j.autrev.2014.01.04624424195 [Google Scholar] [CrossRef] [PubMed]
[11]. Kroll RR, Kumar S, Grossman RF, Price C, Srigley JR, Rare presentation of pulmonary alveolar proteinosis causing acute respiratory failureCanadian Respiratory Journal 2016 2016:406453910.1155/2016/406453927445536 [Google Scholar] [CrossRef] [PubMed]
[12]. Kavuru MS, Malur A, Marshall I, Barna BP, Meziane M, Huizar I, An openlabel trial of rituximab therapy in pulmonary alveolar proteinosisEuropean Respiratory Journal 2011 38(6):1361-67.10.1183/09031936.0019771021478218 [Google Scholar] [CrossRef] [PubMed]