The clinical presentation of haemochromatosis is usually complex and differs from patient to patient. We present one such complex case of primary haemochromatosis in a 35-year-old man with congestive heart failure and non-sustained ventricular tachycardia. He was a known case of diabetes, was infertile after nine years of marriage, and displayed haepatomegaly with increased homogenous attenuation of liver. His serum iron levels (347 μg/dL) and serum ferritin levels (2169 ng/mL) were very high, which made us call him an ‘Iron Man’. Liver biopsy and genetic testing confirmed the diagnosis of primary haemochromatosis. With regular phlebotomies, along with treatments for secondary complications, his symptoms improved gradually and the patient is doing well for past 11 years.
Case Report
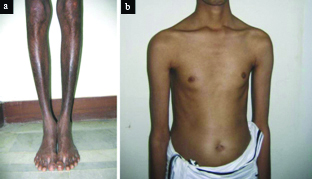
A 35-year-old male patient presented at hospital with acute congestive heart failure. He complained of fever, progressive breathlessness, and hyperpigmentation of lower limbs, and abdominal distension of one week duration [Table/Fig-1a] . He was a known case of diabetes mellitus and was on insulin (Inj. Mixtard 30/70, 8-0-8 units twice daily subcutaneously). He denied history of any blood transfusion or ingestion of iron supplements in the past. His blood pressure was 106/74 mmHg and pulse rate was 76 beats/minute. He was tachypnoeic with a respiratory rate of 26/minute. He complained of dyspnea and restlessness, and his oxygen saturation was 93% on room air. He had elevated jugular venous pressure and bilateral pedal oedema. He exhibited absence of secondary sexual characters (i.e. axillary hair, male eustecheon) and was dealing with infertility nine years after marriage [Table/Fig-1b] . He was diagnosed with hypogonadism and azoospermia and was on monthly testosterone injection as advised by a concerned local area doctor.
Physical examination revealing a) hyperpigmentation of lower limbs and b) absence of secondary sexual characters in a 35-year-old man.
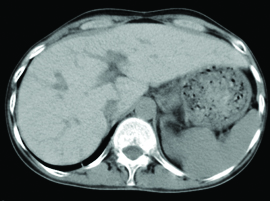
Further, the cardiovascular examination at hospital revealed left as well as right ventricular gallop, chest auscultation revealed bilateral basal crepitations, and abdominal examination revealed palpable liver 8 cm below right costal margin. Subsequently, transthoracic echocardiogram was performed which showed normal left ventricle, mildly dilated right atrium/right ventricle, severe biventricular dysfunction, mild mitral regurgitation, and no pulmonary artery hypertension. He was diagnosed with dilated cardiomyopathy. In addition, the Computed Tomography (CT) scan of abdomen showed hepatomegaly with increased homogenous attenuation of liver and features suggestive of cirrhosis [Table/Fig-2] .
Hepatomegaly with increased homogenous attenuation of liver.
The patient was admitted in critical care unit and was treated initially with ionotropic agents (i.e. dobutamine infusion) and other decongestive measures for congestive heart failure. Electrocardiogram monitoring displayed sinus arrest with junctional escape rhythms at the rate of 25 beats/minute and few runs of non-sustained ventricular tachycardia. During the stay, patient was treated with spirolactone for severe left ventricular systolic dysfunction, but he experienced transient hyperkalemia. Subsequently, he was treated for hyperkalemia with β2 agonists, nebulisation, insulin-glucose infusion, and calcium gluconate injection. After stabilisation, he was shifted to ward.
The patient displayed highly fluctuating blood sugar levels and experienced recurrent episodes of hypoglycemia on even small dose of insulin. He improved symptomatically during the hospital stay and was discharged on eplerenone 50 mg once daily, diuretic (Frusemide, 40 mg once daily), β-blocker (Carvediol, 6.25 mg twice daily), cardiac glycoside (digoxin, 0.25 mg once daily on a 5/7 schedule), antiarrhythmic (amiodarone, 200 mg once daily). Further, the insulin dose was readjusted (Inj. Novomix 30/70, 20-0-8 units subcutaneously). He was also prescribed to 20,000 units of Human Chorionic Gonadotropin (HCG) once a week and 75+75 IU of Follicle Stimulating Hormones (FSH) and Luteinizing Hormone (LH) three times a week for few months for hypogonadism. Additionally, we prescribed further evaluations for haemochromatosis as well as adrenal insufficiency considering the findings from patient’s physical examination, ultrasound examination, and recurrent episodes of hypoglycemia. The patient was advised to seek consultation with these reports after a month.
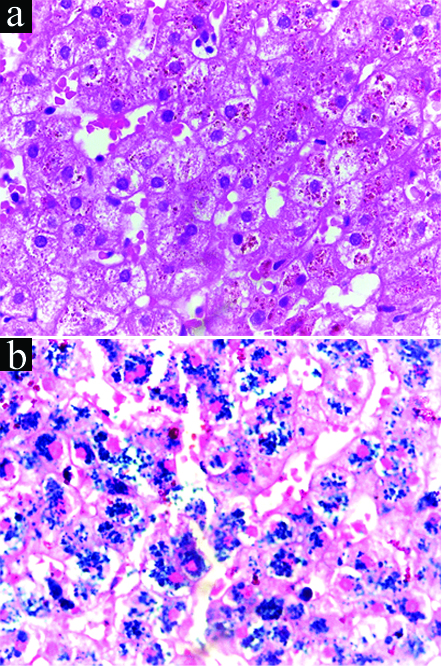
The patient reverted a month later with reports, which revealed very high serum iron and ferritin levels. His serum iron levels were 347 μg/dL (normal range: 55-160 μg/dL in men), serum ferritin levels were 2,169 ng/mL (normal range: 16-300 ng/mL), total iron binding capacity was 350 μg/dL (within normal range) and transferrin saturation was 99%, which made us call him an ‘Iron Man’. His serum cortisol levels were normal while serum testosterone levels were very low (0.057 ng/mL; normal range: 0.1-10.5 ng/mL). The thyroid function tests were normal. We decided to perform liver biopsy to confirm the diagnosis of haemochromatosis. The mononuclear cell infiltrates were characterised in the portal tract and golden-brown pigments were characterised in the perinuclear areas in hepatocytes [Table/Fig-3a] . Moreover, the Perl’s Prussian blue stain was positive, indicating hepatic iron overload [Table/Fig-3b] . Although genetic study was recommended initially, it was not performed due to cost constraints. The patient was advised phlebotomy (300 mL once weekly) for haemochromatosis and testosterone injections (monthly) for hypogonadism. He was kept on regular follow-up.
a) Mononuclear cell infiltrates characterised in the portal tract; b) positive Perl’s Prussian blue stain, indicating hepatic iron overload.
After six months of treatment, transthoracic echocardiogram indicated that patient’s cardiac function had improved to normal. In addition, the serum iron levels were within normal range (74 μg/dL) at two year follow up. Subsequent ultrasonographic examination also showed a normal size of liver (11 cm). The patient was on regular follow-up for past 11 years and was doing well till the last follow-up.
Recently, the patient underwent genetic analysis which showed a rare mutation. There was a single base deletion in exon 4 of Haemochromatosis Type 2 protein (HFE2) gene, formerly known as Haemojuvelin (HJV) gene. In addition, there was a heterozygous truncating variation in exon 19 of MYBPC3 gene on chromosome 11. Accordingly, the variation in HFE2 gene was identified as the disease-causing mutation for hemochromatosis type 2A (i.e. juvenile hemochromatosis), while variation in MYBPC3 gene was identified as the disease-causing mutation for hypertrophic cardiomyopathy.
Discussion
Haemochromatosis is an inherited disorder of iron metabolism [1]. It is characterised by enhanced intestinal absorption, storage, and utilisation of dietary iron, resulting in tissue iron overload [1,2]. The clinical presentation of haemochromatosis is usually complex and differs from patient to patient [2]. Here, we presented one such complex case of primary haemochromatosis and hypertrophic cardiomyopathy in a 35-year-old man with congestive heart failure and non-sustained ventricular tachycardia. Here, the diagnosis of haemochromatosis and hypertrophic cardiomyopathy was substantiated by genetic testing, which was rarely reported in India.
It is well known that iron is one of the most vital mineral required for homeostasis in human system. However, as iron can form free radicals, its concentration in body tissues must be tightly regulated because in excessive amounts, it can lead to tissue damage [3]. In hemochromatosis disorder, an inappropriate increase in intestinal iron absorption results in deposition of excessive amounts of iron in parenchymal cells, which eventually leads to tissue damage and impaired organ function [4]. Without therapeutic management, iron overload may lead to multiple organ damage such as liver cirrhosis, cardiomyopathy, diabetes mellitus, arthritis, hypogonadism, and skin pigmentation [1]. Usually, the onset of severe iron overload occurs typically in the first to third decades of life in patients with haemochromatosis [5]. In contrary to this, significant iron overload was detected at the age of 35 in the present case. The clinical manifestations of primary haemochromatosis are reported in several case reports [6,7]. Ozkurt S et al., reported a case of 61-year-old male patient with primary haemochromatosis, in whom the renal function was deteriorated rapidly [6]. Vogt HJ et al., reported a case of idiopathic haemochromatosis in a 45-year-old infertile man [7].
Hereditary haemochromatosis occurs as a result of HFE gene mutation about 80% cases. In addition, hemojuvelin (HJV), hepcidin (HAMP), Transferrin Receptor 2 (TFR2), and Ferroportin (SLC40A1) are other genes implicated in the pathophysiology of hereditary haemochromatosis [1] Similarly, iron overload cardiomyopathy is associated with genetically determined disorders of iron metabolism [8]. In the present case, genetic testing indicated that mutations in HFE-2 genes were responsible for causing hemochromatosis, while mutation in MYBPC3 gene was linked with pathophysiology dilated cardiomyopathy. Although heart failure may occur as a consequence of haemochromatosis. In the present case, it probably occurred because of another genetic mutation in a gene that resulted in cardiac hypertrophy, indicating that two unusual genetic diseases may coexist. Usually, the dilated cardiomyopathy and haemochromatosis may progress to congestive cardiac failure [5]. Similarly, the indexed patient had presented with congestive heart failure, severe biventricular dysfunction, and non-sustained ventricular tachycardia. He was evaluated for infertility and was found to have low testosterone levels. His serum ferritin and iron levels were very high. The patient subsequently underwent liver biopsy and the diagnosis of haemochromatosis was confirmed.
Early diagnosis and management of haemochromatosis helps in reducing morbidity and mortality in patients. Phlebotomy, a process of removal of one unit of blood (~200 mg of iron) 1-2 times per week for up to 2-3 years, is indicated in majority of patients to reduce iron stores to desired levels. Subsequent phlebotomies are required to maintain iron levels. In addition, patients may require conventional treatments for secondary complications including hypogonadotropic hypogonadism, arthropathy, cardiac failure, liver disease, and diabetes mellitus [5]. The present patient underwent regular phlebotomies along with treatment for cardiac failure, hypogonadotropic hypogonadism, liver disease, and diabetes. His symptoms improved gradually and the patient was doing well for past 11 years.
Conclusion
We strongly believe that genetic testing can lead to more adequate and faster treatment in such patients. In the present case, the diagnosis of haemochromatosis and hypertrophic cardiomyopathy was substantiated by genetic testing. Regular phlebotomies, along with treatments for secondary complications, led to gradual improvements in patients symptoms.
[1]. Santos PCJL, Krieger JE, Pereira AC, Molecular diagnostic and pathogenesis of hereditary hemochromatosisInt J Mol Sci 2012 13(2):1497-511.10.3390/ijms1302149722408404 [Google Scholar] [CrossRef] [PubMed]
[2]. Krysiak R, Okopien B, Autoimmune polyglandular syndrome type 1-like clinical picture of hereditary hemochromatosisPrzegl Lek 2015 72(2):87-89. [Google Scholar]
[3]. Abbaspour N, Hurrell R, Kelishadi R, Review on iron and its importance for human healthJ Res Med Sci 2014 19(2):164-74. [Google Scholar]
[4]. Siah CW, Ombiga J, Adams LA, Trinder D, Olynyk JK, Normal iron metabolism and the pathophysiology of iron overload disordersClin Biochem Rev 2006 27(1):05-16. [Google Scholar]
[5]. Kremastinos DT, Farmakis D, Iron overload cardiomyopathy in clinical practiceCirculation 2011 124(20):2253-64.10.1161/CIRCULATIONAHA.111.05077322083147 [Google Scholar] [CrossRef] [PubMed]
[6]. Ozkurt S, Acikalin MF, Temiz G, Akay OM, Soydan M, Renal hemosiderosis and rapidly progressive glomerulonephritis associated with primary hemochromatosisRen Fail 2014 36(5):814-16.10.3109/0886022X.2014.89239124588645 [Google Scholar] [CrossRef] [PubMed]
[7]. Vogt HJ, Weidenbach T, Marquart KH, Vogel GE, Idiopathic hemochromatosis in a 45-year-old infertile manAndrologia 1987 19(5):532-38.10.1111/j.1439-0272.1987.tb01893.x3122599 [Google Scholar] [CrossRef] [PubMed]
[8]. Juvenile Hereditary Hemochromatosis. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews® [Internet] Seattle (WA): University of Washington, Seattle; 1993-2016. 2005 Feb 17 [updated 2011 Aug 11]. [Google Scholar]