Introduction
The concept of adhesion was introduced into the field of dentistry by Buonocore MG in 1955 [1]. Adhesive dentistry rapidly expanded treatment possibilities and revolutionised the way direct and indirect restorations were traditionally performed. Paralleling the growing demand for adhesive restorations, dentin bonding systems too have undergone an evolution to improve their bond strengths as well as to reduce their technique sensitivity.
Dentin bonding agents have evolved from the gold standard-etch and rinse fifth generation adhesives to the present universal adhesives. The different generations of dentin bonding agents have witnessed a change in chemistry, mechanism of action, procedural steps and a varying degree of clinical efficiency [2]. A recent innovation in the one bottle adhesive systems is their expansion to a more universal bond with 10-Methacryloyloxydecyl Dihydrogen Phosphate (MDP) as the active ingredient. These universal bonding agents can be used in all etch modes for both direct and indirect restorations.
Single bond universal, marketed as Scotchbond Universal in USA, was the first commercial universal adhesive and is popularly used by clinicians worldwide [3-6]. SBU apart from MDP also has methacrylate-modified Polyalkenoic Acid Copolymer (PAAC) in its composition [Table/Fig-1]. Mitra SB et al., reported that PAAC bonds chemically to calcium in hydroxyapatite showing excellent long-term clinical performance thereby, further improving the bond strength [7].
Composition of the bonding agents used in the study.
| Bonding Agent | Composition |
|---|
| Single Bond Universal (3M ESPE) | Methacryloyloxydecyl Dihydrogen Phosphate (MDP), Vitrebond™ copolymer, silane, ethanol-water based solvent |
| Tetric® N-Bond Universal (Ivoclar Vivadent) | Methacrylates (60-70%), water, ethanol (23-28%), highly dispersed silicon dioxide (3-5%), initiators and stabilisers (3-5%) Monomers-Methacryloyloxydecyl dihydrogen phosphate (MDP), Methacrylated carboxylic acid polymer (MCAP), Hydroxyethyl methacrylate (HEMA), Bisphenol A glycidyl methacrylate (Bis-GMA), Decandiol dimethacrylate (D3MA) |
Tetric ®N-Bond Universal is a relatively new universal adhesive which has its matrix based on a combination of monomers of hydrophilic, hydrophobic and intermediate nature allowing it to reliably bridge the gap between the hydrophilic tooth substrate and the hydrophobic restorative resin [Table/Fig-1]. However, studies using this bonding agent are scarce [8-10].
One of the major problems associated with the use of adhesive systems is the difficulty in obtaining a moisture-free clean tooth surface for adequate bonding [11]. Moisture control in the working field is particularly difficult in situations such as equigingival or subgingival cavity margins, seating of indirect restorations, newly erupted molars or when patients have limited mouth opening [12]. Contamination during the bonding process from sources such as gingival crevicular fluid, hand piece oil, blood and saliva, can adversely affect the quality of the bond predisposing it to microleakage at the tooth-restoration interface. As a consequence, loss of the restoration, recurrent caries, postoperative sensitivity and discolouration may occur [13].
Studies in the past have shown that salivary contamination has a deleterious effect on bonding [14-18]. However, manufacturers are claiming that universal bonding agents are resistant to salivary contamination. In accordance to this, study by Santschi K et al., concluded that saliva contamination did not affect the bond strength of SBU [19]. However, Kim J et al., observed that salivary contamination diminishes SBS for universal bonding agents [20]. Also, in the event of contamination, use of an appropriate decontaminating agent to restore bond strengths has been advocated [21,22]. Work by Yoo HM et al., and Santschi K et al., has shown that for all-in-one adhesives, washing, drying and adhesive reapplication was the most effective decontamination protocol [12,19].
Thus, to date, studies which have investigated the effect of salivary contamination on the universal bonding agents are scant and conflicting [19,20]. Hence, the aim of this study was to evaluate the influence of salivary contamination and water rinsing and reapplication of adhesive as a decontamination method on the SBS of universal bonding agents.
Materials and Methods
This in vitro study was done in the Department of Conservative Dentistry and Endodontics, YMT Dental College and Hospital, Navi Mumbai, Maharashtra, India from April 2017-September, 2017.
Sample Selection
Ninety freshly extracted intact, caries free human premolars were selected for the study. Sample size calculation was based on the results (effect sizes) from the previously published study [19]. A sample of size 14 cases in each group, i.e., total 28 cases with the ratio being 1:1 and satisfying the inclusion criteria would produce more than 80.0% statistical power (type II error=0.20) and 5% type I error probability (a=0.05) to be able to detect the clinically important difference in outcome measures between two groups with a two-tailed alternative hypothesis. Hence in the study 15 samples were taken in each subgroup. All the collected teeth were cleared of blood and saliva and cleaned under tap water with a scaler and stored in buffered isotonic saline solution. Teeth with cracks, restorations or any anatomical deformities were excluded from the study.
Sample Preparation and Mounting of Specimens
Teeth were mounted in self-cure acrylic resin (Dental Products India Ltd.,). The occlusal surfaces of the teeth were sectioned off with a double face diamond disc under water cooling to prepare flat dentin surfaces at a depth of 1.5 mm from the cuspal tip of the tooth. The dentin surface to be bonded was ground with #600 SiC paper under running water to produce a standardised smear layer.
Saliva Collection
To achieve standardised salivary contamination, unstimulated human saliva was collected from a single individual at least one hour after any consumption of food or drinks in a sterile beaker and was used immediately.
Division of Samples
Samples were randomly divided into two groups of samples each according to the universal bonding agent used as follows:
Group I- Single Bond Universal (3M ESPE)
Group II- Tetric® N-Bond Universal (Ivoclar Vivadent)
The forty-five premolars in each adhesive group were further divided among three experimental subgroups (n=15) as follows:
Subgroup-1: Control group- The premolars in this group were not subjected to any contamination. The adhesive was applied according to manufacturer’s instructions and light cured for 10 seconds using Bluephase N® LED unit (Ivoclar Vivadent).
Subgroup-2: Contamination group- The adhesive was applied according to manufacturer’s instructions. The specimens were covered with fresh saliva for 20 seconds using a disposable brush. A gentle stream of air was then applied for 2 seconds to dry the surface followed by light curing as in subgroup-1.
Subgroup-3: Decontamination (water rinse and reapplication)- The adhesive was applied according to manufacturer’s instructions. After saliva contamination as in subgroup-2, the contaminated surface was rinsed for 60 seconds with a stream of water from an air-water syringe. A gentle stream of air was then applied for 2 seconds to dry the surface and adhesive was reapplied as a part of decontamination protocol and light cured as in subgroup-1.
Composite Placement
A teflon tube of 3 mm inner diameter and 4 mm length was placed on the surfaces. The Teflon tube was filled with composite resin (Filtek™ Z350, shade A2, 3M ESPE) in two horizontal increments wherein each increment was tightly compressed and light cured for 20 seconds using Bluephase N® LED unit (Ivoclar Vivadent). The teflon tube was removed and the resin cylinder additionally cured.
Preparation of Samples for SBS Analysis
The prepared specimens were stored in distilled water at 37°C for 24 hours and SBS test was carried out using a Universal Testing Machine (UNITEST 10, Acme Engineers, India) at a crosshead speed of 0.5 mm/minute.
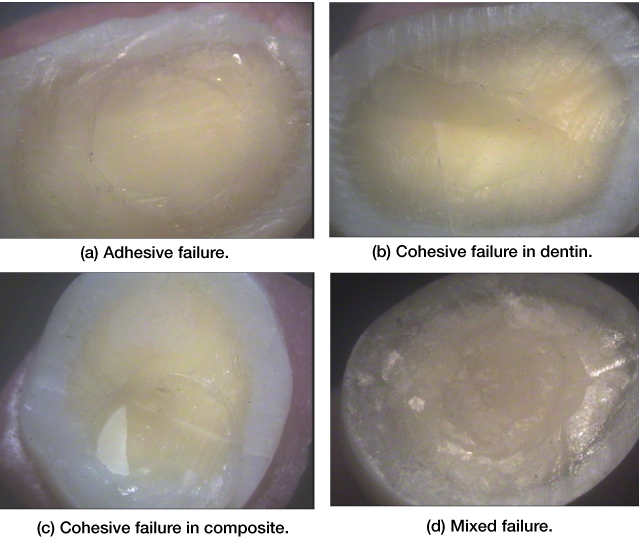
Two examiners evaluated the debonded surfaces at 10X magnification by using a stereomicroscope (Croma Systems) to identify the mode of bond failure (adhesive, cohesive or mixed) [Table/Fig-2].
Stereomicroscopic images (10X magnification) : a) Adhesive failure; b) Cohesive failure in dentin; c) Cohesive failure in composite; d) Mixed failure.
Statistical Analysis
The data obtained in the present study was subjected to statistical analysis using one-way ANOVA test. The intra-group and inter-group comparison was subjected to statistical analysis using Tukeys HSD test (p<0.05). Statistical Package for the Social Sciences (SPSS) software version 17.0 was used for statistical analysis.
Results
Shear bond strength values were obtained for different test groups with SBU and Tetric® N-Bond Universal [Table/Fig-3] and followed by one-way ANOVA test [Table/Fig-4] and Tukey’s HSD test [Table/Fig-5]. A drop in mean SBS was seen after salivary contamination for both the groups. As compared to the contamination group there was an increase in mean SBS in water rinsing group. The intergroup comparison showed that Tetric ®N-Bond Universal group showed significantly better results as compared to SBU group (p<0.05) The mode of failure in all groups was mainly adhesive [Table/Fig-6].
Mean shear bond strengths (in MPa) for each subgroup (Mean±SD).
| Group | Single bond universal | Tetric® N-bond universal |
|---|
| Subgroup-1: Control (n=15) | 18.2 (1.60) | 19.3 (1.69) |
| Subgroup-2: Contamination (n=15) | 13.7 (1.09) | 16.3 (1.03) |
| Subgroup-3: Water rinsing (n=15) | 17.0 (.98) | 18.6 (1.04) |
Statistical analysis using one-way ANOVA test.
| Group | Sum of squares | Mean square | Signature |
|---|
| 1 Group-A | Between Groups | 110.409 | 55.205 | <.001 |
| Within Groups | 42.606 | 1.578 |
| Total | 153.015 | | |
| 2 Group-B | Between Groups | 50.811 | 25.406 | <.001 |
| Within Groups | 45.396 | 1.681 |
| Total | 96.207 | | |
Comparison was subjecte d to statistical analysis using Tukeys HSD test.
| Group | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval |
|---|
| Lower Bound | Upper Bound |
|---|
| Group I- Single bond universal (3M ESPE) | Subgroup-1. Control | Subgroup-2. Contamination | 4.53000* | 0.56178 | <.001 | 3.0750 | 5.9850 |
| Subgroup-3. Decontamination | 1.18300 | 0.56178 | 0.128 | -0.2720 | 2.6380 |
| Subgroup-2.Contamination | Subgroup-3. Decontamination | -3.34700* | 0.56178 | <.001 | -4.8020 | -1.8920 |
| Group II- Tetric ®N-bond universal (Ivoclar Vivadent) | Subgroup-1. Control | Subgroup-2. Contamination | 3.06400* | 0.57989 | 0.000 | 1.5621 | 4.5659 |
| Subgroup-3. Decontamination | 0.77000 | 0.57989 | 0.426 | -0.7319 | 2.2719 |
| Subgroup-2. Contamination | Subgroup-3. Decontamination | -2.29400* | 0.57989 | 0.002 | -3.7959 | -0.7921 |
*The mean difference is significant at the 0.005 level.
| Group | Adhesive | Cohesive in dentin | Cohesive in composite | Mixed |
|---|
| I-1 | 60% (9/15) | 7% (1/15) | 13% (2/15) | 20% (3/15) |
| I-2 | 87% (13/15) | 0 | 0 | 13% (2/15) |
| I-3 | 67% (10/15) | 13% (2/15) | 0 | 20% (3/15) |
| II-1 | 53% (8/15) | 13% (2/15) | 13% (2/15) | 20% (3/15) |
| II-2 | 100% (15/15) | 0 | 0 | 0 |
| II-3 | 73% (11/15) | 13% (2/15) | 0 | 13% (2/15) |
Discussion
The present study was conducted to ascertain both the effect of salivary contamination and decontamination method on SBS of two universal bonding agents-SBU and Tetric® N Bond Universal.
The SBS of dentin was adversely affected by salivary contamination for both the adhesives. Further, statistical analysis revealed that the decontamination protocol had a significant increase in SBS of both the adhesives.
In laboratory tests, the efficacy of dentine adhesion is often evaluated by its SBS. SBS test is useful for a relative comparison of different adhesive systems and for screening new materials [23]. The condition of the substrate that is the tooth structure and the chemical composition of the adhesive system influence the bond strength [24]. As a result, enhancing the efficacy of adhesive restorative materials has been an area of active research.
Pleffken PR et al., and Loguercio AD et al., suggested that active application of the adhesive on dentin improved the bonding performance as well as reduced the degradation rate of the adhesive systems [25,26]. Hence in this study, adhesive was applied to the tooth surface in scrubbing action as instructed by the manufacturer to maximise the bond strength.
During the study on bond strengths of universal bonding agents, Muñoz MA et al., observed that the self etch approach led to more stable bonds even after long-term water storage as against the etch and rinse approach which seemed to be ultra structurally more susceptible to biodegradation over time [27]. Hence in this study, the adhesive was used in self-etch mode.
In the current study, natural human saliva was used as the contaminant. Using artificial saliva or saliva substitutes could have diminished the clinical significance of the study. Moreover, work from several researchers has deemed whole human saliva as an acceptable contaminant [16,19,28,29]. Unstimulated saliva collected from single, healthy individual was used to reduce variability in pH of the saliva and electrolyte, enzyme, or protein content.
In the present study, SBU has consistently shown lower bond strength values as compared to Tetric ®N-Bond Universal. This can be a result of PAAC in SBU competing with MDP by binding to the calcium present in hydroxyapatite. Another possibility could be the prevention of monomer infiltration during polymerisation due to its high molecular weight [30]. However, Awad MM on comparing the same adhesives concluded that, when applied in self-etch mode, both can infiltrate into dentin producing high quality interfacial morphology [8]. Likewise, a study by Jayasheel A et al., comparing SBS of universal adhesives inferred that the bond strength values of the Tetric®N-Bond Universal regardless of application mode were comparable to SBU making them reliable for working under different clinical conditions [9]. Nevertheless, both the above studies were conducted under ideal conditions without taking salivary contamination into consideration.
Saliva is composed mostly of water (99%) with immunoglobulins, polysaccharides, proteins, enzymes and a variety of electrolytes [31]. Researchers have implicated proteins in saliva to be the main factors responsible for reduction in bond strength [14-18,32]. It has been proposed that saliva macromolecules, especially glycoproteins, adsorbed on the enamel surface act as a barrier preventing complete wetting of resin, in turn, inhibiting the monomers from penetrating the collagen network of dentine [33]. Moreover, salivary proteins compete with hydrophilic monomers during the hybridisation process, preventing complete polymerisation of the adhesive, thereby, further reducing bond strength [34,35]. Furthermore, dilution of the adhesive by excess saliva produces a weak hybrid layer.
Vitrebond™ copolymer, the patented product present in SBU is claimed to be moisture tolerant. Despite that we found that the SBS has reduced after salivary contamination. This may be due to adsorption of the biofilm and competition of the monomer during hybridisation [36]. Also, degradation of Bis-GMA due to the hydrolytic enzymes of saliva has been reported which can further compromise bonding [37].
Stage of saliva contamination is also critical towards its effect on bonding [34,38]. In this study specimens were contaminated with saliva after application of bonding agent before light curing. Hence, it evaluated the effect of salivary contamination on the uncured bonding agent as this would directly hamper the formation of hybrid layer. Salivary contamination before polymerisation is particularly significant as Taneja S et al., demonstrated greater decrease in bond strength by contamination at this stage [16]. Moreover, Santschi K has suggested that the bonding agent is highly water soluble which makes it liable to dilution if contamination occurs before polymerization, reducing its bond strength [19].
Water rinsing is an easy choice to combat saliva contamination of a prepared tooth surface. In a study by Sattabanasuk V et al., showed that simply rinsing saliva-contaminated enamel surfaces with water restores the bond strength [32]. On the other hand, studies have demonstrated that conventional washing protocols do not completely remove the coating of salivary proteins on the enamel surface and a subsequent reapplication of the adhesive after water rinsing and air-drying restores bond strength value [39]. This could be attributed to increased resin-dentin interaction due to multiple coatings of adhesive [40-42]. Erickson SO et al., and Cobanoglu N et al., after evaluating several saliva decontamination procedures, proposed application of adhesive after rinsing and drying to be more reliable than just drying, rinsing [31,38]. They suggested that washing and drying should remove the adhesive layer providing a demineralised surface non infiltrated by monomers. Hence, water decontamination followed by reapplication of adhesive was the method of choice used for decontamination in this study.
The type of dentin substrate used could alter also bond strength, as there could be inter-tooth discrepancy and dentinal tubule diameter variation with age and degree of mineralisation [43,44]. These variable factors were overcome by the use of teeth from patients whose ages ranged from 15 to 25 years and within six months of extraction.
Stereoscopic microscopy helped us to evaluate the nature of failure and further gave us an insight in the probable cause for failure. Cohesive mode of failure is persistent if bond strength is more than 20 Mpa [45]. As most of the samples in the study had bond strength less than 20 Mpa, adhesive failure was common in this study.
Limitation
There was limitations in simulating the oral environment in vitro indicating that the excellent physical properties of the adhesive resin that were obtained in vitro are not always attained in vivo. Lower bond strength and failure of adhesives in vivo can be attributed to exposure to oral environment including moisture contact, intraoral temperature, tooth flexure, higher C factor and bacterial enzymes.
Further long term in vitro and in vivo studies are recommended to improve the understanding of the interaction of saliva with various bonding systems which have different chemistry and acidity. Bond durability and sealing ability of the samples decontaminated by the protocol as mentioned in the study after salivary contamination should be investigated. Ongoing research should be directed towards exploration for a novel dentin bonding agent that would be resistant to contamination.
Conclusion
Within the limitations of this in vitro study, it can be concluded that salivary contamination reduces the SBS of universal adhesives to dentin. This is of importance in clinical cases where isolation is a challenge. An additional step of decontamination i.e., reapplication of the adhesive after water rinsing and drying is necessary to regain the bond strength. However, long-term in vivo studies are necessary to substantiate the clinical performance of these adhesive in various clinical situations.
*The mean difference is significant at the 0.005 level.
[1]. Buonocore MG, A simple method for increasing the adhesion of acrylic filling [1]materials to enamel surfacesJ Dent Res 1955 38:849-53.10.1177/0022034555034006080113271655 [Google Scholar] [CrossRef] [PubMed]
[2]. Nair M, Paul J, Kumar S, Chakravarthy Y, Krishna V, Shivaprasad. Comparative evaluation of the bonding efficacy of sixth and seventh generation bonding agents: An in vitro studyJ Conserv Dent 2014 17(1):27-30.10.4103/0972-0707.12411924554856 [Google Scholar] [CrossRef] [PubMed]
[3]. Perdigão J, Sezinando A, Monteiro PC, Laboratory bonding ability of a multi-purpose dentin adhesiveAm J Dent 2012 25(3):153-58. [Google Scholar]
[4]. Perdigão J, Muñoz MA, Sezinando A, Luque-Martinez IV, Staichak R, Reis A, Immediate adhesive properties to dentin and enamel of a universal adhesive associated with a hydrophobic resin coatOper Dent 2014 39(5):489-99.10.2341/13-203-LR24299446 [Google Scholar] [CrossRef] [PubMed]
[5]. Burke FJT, Crisp RJ, Cowan AJ, Raybould L, Redfearn P, Sands P, A randomised controlled trial of a universal bonding agent at three years: Self etch vs total etchEur J Prosthodont Restor Dent 2017 25(4):220-27. [Google Scholar]
[6]. Muñoz MA, Sezinando A, Luque-Martinez I, Szesz AL, Reis A, Loguercio AD, Influence of a hydrophobic resin coating on the bonding efficacy of three universal adhesivesJ Dent 2014 42(5):595-602.10.1016/j.jdent.2014.01.01324508503 [Google Scholar] [CrossRef] [PubMed]
[7]. Mitra SB, Lee CY, Bui HT, Tantbirojn D, Rusin RP, Long-term adhesion and mechanism of bonding of a paste-liquid resin-modified glass-ionomerDent Mater 2009 25(4):459-66.10.1016/j.dental.2008.09.00819041127 [Google Scholar] [CrossRef] [PubMed]
[8]. Awad MM, Assessment of resin-dentin interfacial morphology of two ethanol based universal adhesives: A scanning electron microscopy studyEur J Dent 2017 11(2):206-09.10.4103/ejd.ejd_244_1628729794 [Google Scholar] [CrossRef] [PubMed]
[9]. Jayasheel A, Niranjan N, Pamidi H, Suryakanth MB, Comparative evaluation of shear bond strength of dental adhesives -An in vitro studyJ Clin Exp Dent 2017 9(7):e892-96.10.4317/jced.5381628828156 [Google Scholar] [CrossRef] [PubMed]
[10]. Loguercio AD, Luque-Martinez IV, Fuentes S, Reis A, Muñozn MA, Effect of dentin roughness on the adhesive performance in non-carious cervical lesions: a double-blind randomized clinical trialJ Dent 2017 S0300-5712(17):30237-33.10.1016/j.dental.2017.08.098 [Google Scholar] [CrossRef]
[11]. Xie J, Powers JM, McGuckin RS, In vitro bond strength of two adhesives to enamel and dentin under normal and contaminated conditionsDent Mater 1993 9(5):295-99.10.1016/0109-5641(93)90046-S [Google Scholar] [CrossRef]
[12]. Yoo HM, Oh TS, Pereira PN, Effect of saliva contamination on the micro shear bond strength of one step-etching Adhesive Systems to DentinOper Dent 2006 31:127-34.10.2341/04-20616536204 [Google Scholar] [CrossRef] [PubMed]
[13]. Swift EJ, Perdigão J, Heymann HO, Bonding to enamel and dentin: a brief history and state of the art, 1995Quintessence Int 1995 26(2):95-110. [Google Scholar]
[14]. Guo HJ, Guo CZ, Lin F, Liu W, Yue L, Effects of saliva contamination on bond strength of resin-resin interfacesBeijing Da Xue Xue Bao Yi Xue Ban 2017 49(1):96-100. [Google Scholar]
[15]. Gupta N, Tripathi AM, Saha S, Dhinsa K, Garg A, Effect of saliva on the tensile bond strength of different generation adhesive systems: an in vitro studyJ Clin Diagn Res 2015 9(7):ZC91-ZC94.10.7860/JCDR/2015/13801.625126393214 [Google Scholar] [CrossRef] [PubMed]
[16]. Taneja S, Kumari M, Bansal S, Effect of saliva and blood contamination on the shear bond strength of fifth-, seventh-, and eighth-generation bonding agents: An in vitro studyJ Conserv Dent 2017 20(3):157-60.10.4103/0972-0707.21831029279617 [Google Scholar] [CrossRef] [PubMed]
[17]. Munaga S, Chitumalla R, Kubigiri SK, Rawtiya M, Khan S, Sajjan P, Effect of saliva contamination on the shear bond strength of a new self-etch adhesive system to dentinJ Conserv Dent 2014 17(1):31-34.10.4103/0972-0707.12412424554857 [Google Scholar] [CrossRef] [PubMed]
[18]. Koppolu M, Gogala D, Mathew VB, Thangala V, Deepthi M, Sasidhar N, Effect of saliva and blood contamination on the bond strength of self-etching adhesive system- An in vitro studyJ Conserv Dent 2012 15(3):270-73. 10.4103/0972-0707.9795622876017 [Google Scholar] [CrossRef] [PubMed]
[19]. Santschi K, Peutzfeldt A, Lussi A, Flury S, Effect of salivary contamination and decontamination on bond strength of two one-step self-etching adhesives to dentin of primary and permanent teethJ Adhes Dent 2015 17(1):51-57. [Google Scholar]
[20]. Kim J, Hong S, Choi Y, Parkn S, The effect of saliva decontamination procedures on dentin bond strength after universal adhesive curingRestor Dent Endod 2015 40(4):299-305.10.5395/rde.2015.40.4.29926587416 [Google Scholar] [CrossRef] [PubMed]
[21]. Juneja R, Duhan J, Tewari S, Sangwan P, Bhatnagar N, Effect of blood contamination and decontamination protocols on acetone-based and ethanol- based total etch adhesive systemsJ Esthet Restor Dent 2014 26(6):26-16.10.1111/jerd.1208924417739 [Google Scholar] [CrossRef] [PubMed]
[22]. Ülker E, Bilgin S, Kahvecioğlu F, Erkan AI, Effect of saliva decontamination procedures on shear bond strength of a one-step adhesive systemNiger J Clin Pract 2017 20(9):1201-05.10.4103/1119-3077.18732529072247 [Google Scholar] [CrossRef] [PubMed]
[23]. Park J, Lee KC, The influence of salivary contamination on shear bond strength of dentin adhesive systemsOper Dent 2004 29(1):437-42. [Google Scholar]
[24]. Van Meerbeek BV, Inoue S, Perdigao S, Lambrechts P, Vanherle G, Enamel [24]and dentin adhesion. In: Summitt JB, Robbins JW, Schwartz RS, editors 2000 2nd EditionChicago: Illinios Quintessence Publishing Co IncFundamentals of Operative Dentistry:178-221. [Google Scholar]
[25]. Pleffken PR, de Almeida Lourençoa AP, Torres CR, Bühler Borgesb A, Influence of application methods of self-etching adhesive systems on adhesive bond strength to dentinJ Adhes Dent 2011 13(1):517-25. [Google Scholar]
[26]. Loguercio AD, Stanislawczuk R, Mena-Serrano A, Reis A, Effect of 3-year water [26]storage on the performance of one-step self-etch adhesives applied actively on dentineJ Dent 2011 39(8):578-87.10.1016/j.jdent.2011.06.00521726597 [Google Scholar] [CrossRef] [PubMed]
[27]. Muñoz MA, Luque-Martinez I, Malaquias P, Hass V, Reis A, Campanha NH, In vitro longevity of bonding properties of universal adhesives to dentinOper Dent 2015 40(3):282-92.10.2341/14-055-L25405904 [Google Scholar] [CrossRef] [PubMed]
[28]. Yazici AR, Tuncer D, Dayangaç B, Ozgünaltay G, Onen A, The effect of saliva contamination on microleakage of an etch-and-rinse and a self-etching adhesiveJ Adhes Dent 2007 9(1):305-09. [Google Scholar]
[29]. Q Jiang, H Pan, B Liang, B Fu, M Hannig, Effect of saliva contamination and [29]decontamination on bovine enamel bond strength of four self-etching adhesivesOper Dent 2010 35(1):194-202.10.2341/09-151-L20420063 [Google Scholar] [CrossRef] [PubMed]
[30]. Y Yoshida, K Yoshihara, N Nagaoka, S Hayakawa, Y Torii, T Ogawa, Selfassembled Nano-layering at the Adhesive interfaceJ Dent Res 2012 91(4):376-81.10.1177/002203451243737522302145 [Google Scholar] [CrossRef] [PubMed]
[31]. SO Eiriksson, PN Pereira, EJ Swift, HO Heymann, A Sigurdsson, Effects of saliva contamination on resin-resin bond strengthDent Mater 2004 20(1):37-44.10.1016/S0109-5641(03)00066-6 [Google Scholar] [CrossRef]
[32]. Sattabanasuk V, Shimada Y, Tagami J, Effects of saliva contamination on dentin bond strength using all-in-one adhesivesJ Adhes Dent 2006 8(5):311-18. [Google Scholar]
[33]. Abdalla AI, Davidson CL, Bonding efficiency and interfacial morphology of onebottle adhesives to contaminated dentin surfacesAm J Dent 1998 11:281-85. [Google Scholar]
[34]. Neelagiri K, Kundabala M, Shashi RA, Thomas MS, Parolia A, Effects of saliva contamination and decontamination procedures on shear bond strength of selfetch dentine bonding systems: An in vitro studyJ Conserv Dent 2010 13(2):71-75.10.4103/0972-0707.6671420859478 [Google Scholar] [CrossRef] [PubMed]
[35]. Kermanshah H, Ghabraei SH, Bitaraf T, Effect of salivary contamination during different bonding stages on shear dentin bond strength of one-step self-etch and total etch adhesiveJ Dent (Tehran) 2010 7(3):132-38. [Google Scholar]
[36]. Hashimoto M, Tay FR, Svizero NR, de Gee AJ, Feilzer AJ, Sano H, The effects of common errors on sealing ability of total-etch adhesivesDent Mater 2006 22:560-68.10.1016/j.dental.2005.06.00416289724 [Google Scholar] [CrossRef] [PubMed]
[37]. Hiraishi N, Kitasako Y, Nikaido T, Nomura S, Burrow MF, Tagami J, Effect of artificial saliva contamination on pH value change and dentin bond strengthDent Mater 2003 19:429-34.10.1016/S0109-5641(02)00087-8 [Google Scholar] [CrossRef]
[38]. Cobanoglu N, Unlu N, Ozer FF, Blatz MB, Bond strength of self-etch adhesives after saliva contamination at different application stepsOper Dent 2013 38(5):505-11.10.2341/12-260-L23327232 [Google Scholar] [CrossRef] [PubMed]
[39]. Silverstone LM, Hicks MJ, Featherstone MJ, Oral fluid contamination of etched enamel surfaces: An SEM studyJ Am Dent Assoc 1985 110(3):329-32.10.14219/jada.archive.1985.03503889092 [Google Scholar] [CrossRef] [PubMed]
[40]. Hashimoto M, Sano H, Yoshida E, Hori M, Kaga M, Oguchi H, Effects of multiple adhesive coatings on Dentin bondingOper Dent 2004 29:416-23. [Google Scholar]
[41]. Albuquerque M, Pegoraro M, Mattei G, Reis A, Loguercio AD, Effect of double application of a hydrophobic layer for improved efficacy of One-step Selfetch system in enamel and dentinOper Dent 2008 33(1):564-70.10.2341/07-14518833863 [Google Scholar] [CrossRef] [PubMed]
[42]. Ito S, Tay FR, Hashimoto M, Yoshiyama M, Saito T, Brackett WW, Effects of multiple coatings of two all-in-one adhesives on dentin bondingJ Adhes Dent 2005 7:133-41. [Google Scholar]
[43]. Burrow MF, Harada N, Kitasako Y, Nikaido T, Tagami J, Seven year dentin bond strengths of a total and self etch systemEur J Oral Sci 2005 113(3):265-70.10.1111/j.1600-0722.2005.00213.x15953253 [Google Scholar] [CrossRef] [PubMed]
[44]. Bayne SC, Thompson JY, Taylor DF, Dental materials chapter 4, Sturdevent’s art and science of operative dentistry 2002 4th Edition:190-207. [Google Scholar]
[45]. Bouillaguet S, Gysi P, Wataha JC, Ciucchi B, Cattani M, Godin C, Bond strength of composite to dentin using conventional, one-step, and self-etching adhesive systemsJ Dent 2001 29(1):55-61.10.1016/S0300-5712(00)00049-X [Google Scholar] [CrossRef]