Introduction
Diabetes is a complex chronic disease associated with rise in plasma glucose level, due to dysfunction in insulin release, insulin action or both. Prevalence of T2DM is increasing and approaching towards most common non communicable disease worldwide [1]. According to WHO report 2016, around 422 million people worldwide lived with diabetes in 2014 compared to 108 million in 1980. Over the past decade, the prevalence of diabetes increases in lower and middle income countries [1]. With 79.9 million adult people with T2DM, India ranked second in diabetes, behind China as per the International Diabetic Federation (IDF) report. IDF also projected that Indian diabetic population may cross 134.3 million by 2045 [2].
Hyperglycaemia is an inherent factor in diabetes. It leads to the development of micro and macrovascular complications. Two essential factors are readily involved in hyperglycaemia; insulin resistance and β cells dysfunction. In the initial phase, β cells cope up with an increase in blood glucose level by substantial release of insulin from β cells. When β cells are unable to produce enough insulin to overcome the excessive blood glucose level, it leads to hyperglycaemia [3]. It may increase morbidity and mortality in diabetes patients.
Macrovascular complications in T2DM patients are more common, about 65% of patients die due to Cardiovascular Disease (CVD), almost 80% due to coronary artery disease [4]. The American Diabetic Association (ADA) recommended target level of HbA1c is <7%, whereas by American Association of Clinical Endocrinologist (AACE) it is ≤6.5% in diabetes [5,6]. Fasting Plasma Glucose (FPG) and PPG are two important parameters for glucose estimation [7]. Recent clinical evidence strongly suggested that postprandial hyperglycaemia control may be necessary for HbA1c achievement [8]. Negligence of PPG level may increase the risk of CVD [9].
The primary goal of management in T2DM is to improve quality of life and good metabolic control. Pharmacological intervention is required when lifestyle modifications are not able to maintain normal plasma glucose. Selection of pharmacological therapy is based on efficacy, tolerability, effects on comorbid conditions, hypoglycaemia risk, cost and patient preferences [10].
The α-Glucosidase Inhibitors (AGI) have been categorised as oral hypoglycaemic agents. These drugs delay the breakdown of oligosaccharides by competing with enzyme α-glucosidase. It delays the absorption of glucose, subsequently reduces the postprandial glucose level [11]. Voglibose, one of the potent and tolerant drug in class of AGIs. It was first discovered in Japan in 1981 and commercially available in year 1994 for the treatment of T2DM [12]. Treatment with voglibose increases the level of Glucagon Like Peptide-1 (GLP-1), which is known for increase in insulin secretion [13]. Voglibose is effective as monotherapy or in combination with other antidiabetic medications. The primary objective of this article was to review current clinical evidences of voglibose in the management of PPG in T2DM patients, its preventive action and cardiovascular benefits.
Materials and Methods
Extensive search work was conducted on Google scholar and PubMed database from January, 1990 to May, 2017. Keywords used for search was; Voglibose, α-glucosidase inhibitors, diabetes mellitus and postprandial hyperglycaemia. Search included all research articles, review articles, meta-analysis, guidelines and textbooks. Approximately 310 articles on voglibose were scanned from different databases. Multiple publications addressing the same dataset have been filtered out. Animal studies, in vivo, in vitro studies and articles in language other than English were excluded. Twenty four potential articles of voglibose on clinical efficacy, cardiovascular effect, pre diabetic action, safety and pharmacokinetics have been incorporated in this article.
Importance of PPG in management of Type 2 diabetes mellitus
PPG, FPG and HbA1c are important for optimal glycaemic control. However, which value is more important is still questionable [14]. PPG is a pernicious factor in the development of diabetes and related complications [15,16]. Current ADA criteria for the diagnosis of PPG is plasma glucose level ≥200 mg/dL [5]. A meta-analysis on correlation of HbA1c with FPG and PPG highlighted the closer association of PPG with HbA1c compare to FPG; hence, PPG is better in predicting overall glycaemic control in the absence of HbA1c [17]. The Japanese study highlighted that the correlation of HbA1c was better with PPG than FPG. In fact PPG level measured after breakfast and dinner had shown the strongest relation with HbA1c [18]. In another study conducted by Monnier L et al., involving 290 patients, has highlighted the PPG level has greater impact on HbA1c in patients with good glycaemic control and FPG levels has greater impact in patients with poor glycaemic control [19]. A study by Bonora E et al., on T2DM, concluded that more than 60% of patients had higher PPG level (>160 mg/dL) even with HbA1c less than 7 in same population [20]. Holman RR et al., confirms the PPG control makes a greater contribution towards glycaemic control in subjects with normal range of HbA1c value [21].
Cardiovascular Complications and PPG
T2DM considered as one of the major independent risk factor for CVD. Even in non diabetic patients, the level of blood glucose has an impact on cardiovascular risk [22]. In hyperglycaemia, endothelial dysfunction and oxidative stress are important marker for cardiovascular complications. Production of free radical increases the oxidative stress and leads to cardiovascular complications. For the prevention of cardiovascular complication in diabetic patients, correction of PPG level is utmost important [17]. Based on different epidemiological and interventional studies, it can be concluded that postprandial hyperglycaemia is mainly responsible for complications, especially CVD in T2DM [Table/Fig-1] [16,23-28].
Cardiovascular disease and mortality associated with postprandial hyperglycaemia [16,23-28].
| Study | Interpretation |
|---|
| PPG: A risk factor for increased CIMT in non diabetic individuals [16] | Post meal hyperglycaemia exerts a harmful impact on carotid intima media thickness |
| Hoorn Study [23] | 2 hour post load glucose level is a better predictor of risk of all-cause and cardiovascular mortality than HbA1c |
| Honolulu Heart Program [24] | Linear relation noted between post challenge glucose and fatal Congestive Heart Disease (CHD) and non-fatal Myocardial Infarction (MI) |
| Chicago Heart Association Study [25] | High PPG level increases risk of CVD and mortality |
| Diabetes Intervention Study, 11 years follow-up [26] | The post meal glucose level is associated with CHD |
| DECODE Study [27] | High 2 hour post load blood glucose provide additional information who have the greater risk of death, independent of fasting glucose |
| Incident cardiovascular events and glucose level: A meta regression analysis [28] | 2 hour glucose associated with cardiovascular event risk |
PPG- Postprandial glucose; CVD-Cardiovascular disease; DECODE- Diabetes epidemiology: collaborative analysis of diagnostic criteria in Europe
Management of PPG
International guidelines have different recommendations on PPG goal. IDF recommends <160 mg/dL, AACE recommends <140 mg/dL and ADA suggests <180 mg/dL [29]. Drugs, including short-acting Sulfonylureas (SU), Dipeptidyl Peptidase-4 (DPP4) inhibitors, AGIs, GLP-1 derivatives, glinides, and rapidly acting human insulin or insulin analogues are common medications which focus on PPG treatment as mentioned in [Table/Fig-2] [30].
Classification and mode of action of antidiabetic agents with PPG as potential target [31].
GLP-1-Glucagon like peptide-1; DPP4-Dipeptidyl peptidase-4; PPG-Postprandial glucosein
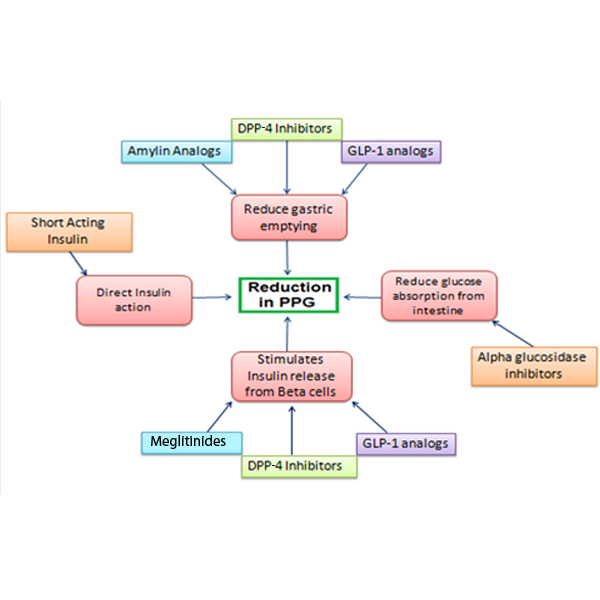
Developing countries like China and India, consume carbohydrate rich diet, accounting 80% of daily calorie [31]. In a study on Indian population, high carbohydrate intake increases the triglyceride level in both normal and diabetes population [32]. Targeting the glucose control from carbohydrate rich diet is an effective way for PPG control.
Voglibose, in Management of T2DM
Voglibose belongs to category of α-glucosidase inhibitors. Voglibose delays glucose absorption from intestine by inhibiting α-glucosidase enzyme. As a result it prevents high blood glucose level and insulin spike after meal [33]. Voglibose is available in oral tablets, disintegrating tablets or in sustained release tablets as single or in combination with other antidiabetic drugs. Usual dose of voglibose is 0.2 mg with meal; it can be increased to 0.3 mg if necessary [33]. Recently published AACE/ACE guideline for management of T2DM, emphasises the role of voglibose as monotherapy after lifestyle modifications [6]. The Japanese guideline also advocates voglibose as a first line of therapy in people with diabetes, having high postprandial glucose level [34]. However, ADA recommends voglibose as a second line of therapy with metformin combination in T2DM patients. ADA also stated the use of voglibose in pre diabetic conditions [13].
Mechanism of action: α-glucosidase enzymes are located in the epithelial cell lining of intestine, specifically in enterocytes. The role of these enzymes is conversion of polysaccharides and oligosaccharide glucosein simple glucose so that they can be easily absorbed from intestine and reaches the blood circulation. These monosaccharides are absorbed readily via both specific (Na+ dependent active transport and facilitated diffusion) and non-specific (passive diffusion) transport routes. Voglibose prevents the actions of these enzymes, results in delay in digestion and absorption of carbohydrates and results in impairment of blood glucose spikes. It is a more specific inhibitor of disaccharides digesting enzymes such as sucrase and maltase and has minimal effect on alpha-amylase. Voglibose does not have secretagogues action on pancreatic β-cells for insulin release and hence does not cause hypoglycaemia [33].
Voglibose is poorly absorbed; majority of active ingredients remains unchanged and remains in the gastrointestinal tract. Plasma concentration generally not detectable, its bioavailability is less than 6%. Excretion of voglibose occurs through faeces and negligible renal excretion. No drug interaction has been reported with concomitant administration with digoxin, dapagliflozin, glibenclamide, hydrochlorothiazide, metformin, vildagliptin, or warfarin [33]. In case of co-adminstration with vildagliptin, voglibose shows synergistic effect on activation of GLP-1 plasma concentration than vildagliptin alone [35]. When voglibose administered with dapagliflozin, it slightly increases half-life of dapagliflozin [36].
Clinical Efficacy of Voglibose in Management of T2DM
Voglibose has been studied as monotherapy or in combination with other oral antidiabetic medications [Table/Fig-3] [37-43]. As a monotherapy, it has been compared with drugs like acarbose, miglitol, linagliptin and sitagliptin. Based on the results, voglibose had mild glucose lowering efficacy; however, its effects on PPG level is superior.
Clinical studies of voglibose as a monotherapy in T2DM management [37-43].
| Study Design, Duration and number of Patients | Study Group (Gr) | Results | Conclusion |
|---|
| Lee MY et al., Multicentric, randomised, open label. Duration: 24 weeks, n=121 [37] | Gr I: Voglibose Gr II: Acarbose | PPG reduction (mg/dL) Gr I: -33.52±73.24 (p<0.001) Gr II: -55.99±68.93 (p<0.05) | Both Voglibose and Acarbose are efficacious in T2DM patients. Gastrointestinal adverse events are more in Acarbose group. |
| Ismail D and Deshmukh S, Single blind, randomised, comparative and prospective clinical trial. Duration: 6 months; n=90 [38] | Gr I: Miglitol Gr II: Acarbose Gr III: Voglibose | PPG reduction (mg/dL) Gr I: - 86.63 (p<0.001) Gr II: - 58.46 (p<0.001) Gr III: - 93.70 (p<0.001) | All three are effective in PPG reduction, but Voglibose is better than Acarbose and miglitol in terms of safety |
| Fujitani Y et al., Multicenter, randomised; 12 weeks; n=382 [39] | Gr I: Linagliptin Gr II: Voglibose | Mean serum glucose conc. reduction (mmol/L) Gr I: - 1.98±1.88 Gr II: -2.45±1.88 (p=0.019) | Mean serum glucose conc. reduction and body weight reduction is significantly more with Voglibose than Linagliptin. |
| Seo C et al., Crossover study; 8 weeks; n=17 [40] | Gr I: Voglibose Gr II: Sitagliptin | Glucose elevation after meal (mg/dL/minute) Breakfast: Gr I: 0.86; Gr II: 1.16 (p<0.05) Lunch: Gr I: 0.45; Gr II: 0.70 (p<.05) Dinner: Gr I: 0.73; Gr II: 1.06 (p<0.05) | Significantly better control on glucose elevation after meal in Voglibose group. |
| Vichayanrat A et al., randomised crossover study; 8 weeks; n=30 [41] | Gr I: Voglibose Gr II: Acarbose | 1 hour PPG reduction At 4 weeks reduction from baseline Gr I: 20.8 (p=0.005) Gr II: 45.6 (p<0.001) | Voglibose and Acarbose are equally effective on PPBG reduction, but Voglibose has less adverse drug events compare to Acarbose |
| Woo JT et al., multicentre open study, 8 weeks; n=55 [42] | Voglibose+Diet | Serum glucose level at 1 hour (mmol/L) At 0 week: 17.5 At 4 weeks: 15.4 (p<0.001) At 8 weeks: 14.8 (p<0.001) | Voglibose monotherapy effective on postprandial glucose level in T2DM patients whose postprandial glucose cannot be achieved with diet control only. |
| Iwamoto Y et al., multicenter, randomised, double blind; 12 week; n= 319 [43] | Gr I: Sitagliptin Gr II: Voglibose | Change 2 hour Post meal glucose (mmol/L) Gr I: 2.8 Gr II: 1.8 | Sitagliptin showed better efficacy both on 2 hour post meal glucose compare to Voglibose. |
T2DM: Type 2 diabetes mellitus, PPBG: Postprandial blood glucose, PPG: Postprandial glucose
Limited data with small patient population are available in combination of voglibose with other antidiabetic medications. Most common combination of voglibose is along with mitiglinide which targets postprandial plasma glucose level. Ohta A et al., compared voglibose and mitiglinide combination to sitagliptin and concluded that combination achieve better glycaemic control over sitagliptin therapy [44]. In another 12 weeks study by Katsuno T et al., on using voglibose and mitiglinide combination resulted better PPG control than dual dose of mitiglinide [45]. In a randomised crossover study, voglibose and mitiglinide as monotherapy and combination of both has been evaluated. The results showed the better postprandial glucose control with combination than in montherapies [46]. In a combination of voglibose with SU, significantly better control of FPG and PPG level were reported [47]. More data with larger patient population is needed for establishment of efficacy and safety of combination therapy.
Role of Voglibose on Cardiovascular Events in T2DM Patients
Oxidative stress, endothelial dysfunction and inflammation play an important role in CVD. These markers increase with high level of PPG. A STOP NIDDM trial has proven the reduction of cardiovascular events and hypertension by reducing PPG level [48]. In a comparative study between voglibose and sitagliptin, it was found that voglibose improved the endothelial dysfunction [49]. A study on voglibose resulted the reduction of progression of intima media thickness [50]. A study on obese T2DM patients, treated with diet alone and diet and voglibose, highlighted that voglibose decreases the plasma levels of soluble intercellular adhesion molecule 1 and urinary excretion of 8-iso-prostaglandin F(2) alpha and 8-hydroxydeoxyguanosine (p<0.01) and C-reactive protein (p<0.05), which are marker of oxidative stress [51]. In another study which was conducted on patients with T2DM and metabolic syndrome, compared pioglitazone and voglibose for period of six months follow-up. After follow-up period, basal metabolic index and waist circumference significantly decrease in voglibose group, but remain unchanged in pioglitazone group [52].
Role in Pre-diabetes Patients
Prediabetic condition or Impaired Glucose Tolerance (IGT) is a possible explanation for rising risk of coronary artery disease. Patients with deteriorating glucose tolerance have the higher risk of macrovascular diseases. Based on the finding of Honolulu [24] and Islington heart studies [53], it was found that mortality increases with increase in deterioration of glucose tolerance. A Japanese study was also conducted by Kawamori R et al., to know whether voglibose would be able to prevent T2DM in patients with IGT [54]. This study was conducted on 1780 patients, where voglibose was compared with placebo and study results demonstrated 40.5% risk reduction for T2DM as compared to placebo. Significantly more subjects achieve normoglycaemia condition in voglibose group. An opinion based Indian study by Dewda PR and Agrawal S with 117 practitioners, was conducted on use of medication in case of prediabetes [55]. Based on the doctor’s response, voglibose is considered as most favoured second line drug in prediabetic condition. The first preference was metformin.
Adverse Events
Unabsorbed carbohydrate and glucose gets accumulated in intestine that may cause some of side effects. Most commonly reported side effects with voglibose are gastrointestinal related. It includes soft stools or diarrhoea, flatulence, bloating, abdominal pain or discomfort, abdominal fullness and nausea. In a study involving 1780 patients with impaired glucose tolerance, the incidence of treatment related adverse events were 48% with voglibose and 29% with placebo. Flatulence, abdominal distension, diarrhoea, abnormal bowel sound were the most common side effects with voglibose, but all are mild to moderate [33].
Current Place in Therapy and Expert Comment on its Clinical Utility
There is established fact that with an increase in plasma glucose level, the chances of micro and macrovascular complications are increased. The traditional concept of management of T2DM is based on level of fasting plasma glucose, although there was no evidence on relation between FPG and morbidity or mortality. Even in many cases, HbA1c achieve optimal level, but PPG not reaching to goal. This leads to increase in other complications [7]. Studies like EPIC-Norfolk, Atherosclerosis Risk in Communities (ARIC) study and UKPDS Study showed an inverse correlation between hyperglycaemia and CVD [21]. Oxidative stress and endothelial dysfunction, plays an important mechanism for CVD in patients with high PPG [50,51]. Based on evidences, now PPG is an important target to control complications in diabetes patients.
Voglibose was developed by Takeda pharmaceutical in Japan in year 1981. Initally, it was isolated from Streptomyces hygroscopicies var. limonons [10]. It can be used as a first line of therapy in patients with high PPG level or as add-on therapy with other potent oral antidiabetic medications such as metformin or SU. It is available in single pill or in fixed dose combinations with other oral hypoglycaemic agents. Available studies on voglibose as monotherapy, demonstrated its efficacy on PPG and other parameters compared to that of other oral hypoglycaemic agents. Beyond the glycaemic control, it has a cardioprotective effect by exhibiting strong suppression of PPG. Overall voglibose is a drug of choice, particularly in individuals with an elevated postprandial glucose level and it also has a cardioprotective benefit.
Conclusion
Voglibose is a potential therapeutic option for patients with T2DM with high PPG level. It also improves the PPG level in patients who are poorly controlled with other hypoglycaemic agents even with optimal HbA1c level. Voglibose have ample of clinical evidences on PPG level as monotherapy or in combination with other oral antidiabetic drugs. Effective control of PPG level helps in prevention of cardiovascular outcomes. Beyond the glycaemic control, it has cardioprotective activity by controlling oxidative stress and endothelial dysfunction. Voglibose is effective and it is generally well tolerated in a wide range of T2DM patients. Voglibose is useful in elderly patients and in those with hepatic impairment or mild to moderate renal function impairments when other anti-diabetic agents are contraindicated.
Conflict of interest: Mr. Neeraj Kumar and Dr. Onkar C Swami are full time employees of Torrent Pharmaceuticals Limited, which actively markets Voglibose. The other authors report no conflicts of interest in this work.
[1]. World Health Organization. Global report on diabetes. 2016. [Cited 2017 8 Aug.] Available from : http://www.who.int/diabetes/global-report10.4103/2468-8827.184853 [Google Scholar] [CrossRef]
[2]. International Diabetes Fedration. IDF diabetes atlas, 8th ed. International Diabetes [2]Federation; 2017. [Cited 2018 5 Jan.]. Available from: http://www.diabetesatlas.org/resources/2017-atlas.html [Google Scholar]
[3]. Campos C, Chronic hyperglycaemia and glucose toxicity: pathology and clinical sequelaePostgraduate Medicine 2012 124(6):90-97.10.3810/pgm.2012.11.261523322142 [Google Scholar] [CrossRef] [PubMed]
[4]. Unnikrishnan R, Anjana RM, Mohan V, Diabetes mellitus and its complications in IndiaNat Rev Endocrinol 2016 12(6):357-70.10.1038/nrendo.2016.5327080137 [Google Scholar] [CrossRef] [PubMed]
[5]. American Diabetes Association. Glycaemic targets. Sec. 6. In Standards of Medical Care in Diabetes-2017Diabetes Care 2017 40(Suppl.1 1):S48-S56.10.2337/dc17-S00927979893 [Google Scholar] [CrossRef] [PubMed]
[6]. Garber AJ, Abrahamson MJ, Barzilay Ji, Blonde L, Bloomgarden ZT, Bush MA, Consensus statement by the American Association of Clinical Endocrinologists and American college of Endocrinology on the Comprehensive type 2 diabetes management algorithm-2017 executive summaryEndocrine Practice 2017 23(2):207-38.10.4158/EP161682.CS28095040 [Google Scholar] [CrossRef] [PubMed]
[7]. Ozmen S, Cil T, Atay AE, Tuzcu AK, Bahceci M, A simple way to estimate mean plasma glucose and to identify Type 2 diabetes subjects with poor glycaemic control when standardized HbA1c assay is not availableDiabet Med 2006 23(10):1151-54.10.1111/j.1464-5491.2006.01927.x16978383 [Google Scholar] [CrossRef] [PubMed]
[8]. Woerle HJ, Neumann C, Zschau S, Tenner S, Irsgler A, Schirra J, Impact of fasting and postprandial glycaemia on overall glycaemic control in type 2 diabetes Importance of postprandial glycaemia to achieve target HbA1c levelsDiabetes Res Clin Pract 2007 77(2):280-85.10.1016/j.diabres.2006.11.01117240473 [Google Scholar] [CrossRef] [PubMed]
[9]. Aryangat AV, Gerich JE, Type 2 diabetes: postprandial hyperglycaemia andincreased cardiovascular riskVasc Health Risk Manag 2010 6:145-55.10.2147/VHRM.S821620448799 [Google Scholar] [CrossRef] [PubMed]
[10]. Aravind SR, Saboo B, Sadikot S, Shah SN, Makkar BM, Kalra S, Consensus statement on management of post-prandial hyperglycaemia in clinical practice in IndiaJ Assoc Physicians India 2015 63(8):45-58. [Google Scholar]
[11]. Joshi SR, Standl E, Tong N, Shah P, Kalra S, Rathod R, Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based reviewExpert Opin Pharmacother 2015 16(13):1959-81.10.1517/14656566.2015.107082726255950 [Google Scholar] [CrossRef] [PubMed]
[12]. Dabhi AS, Bhatt NR, Shah MJ, Voglibose: an alpha glucosidase inhibitorJ Clin Diagn Res. 2013 7(12):3023-27. [Google Scholar]
[13]. Goke B, Fuder H, Wieckhorst G, Theiss U, Stridde E, Littke T, Voglibose (AO-128) is an efficient alpha-glucosidase inhibitor and mobilizes the endogenous GLP-1 reserveDigestion 1995 56(6):493-501.10.1159/0002012828536820 [Google Scholar] [CrossRef] [PubMed]
[14]. Schrot RJ, Targeting plasma glucose: preprandial versus postprandialClinical [14]Diabetes 2004 22(4):169-72.10.2337/diaclin.22.4.169 [Google Scholar] [CrossRef]
[15]. Ceriello A, Cardiovascular effects of acute hyperglycaemia: pathophysiological underpinningsDiab Vasc Dis Res 2008 5(4):260-68.10.3132/dvdr.2008.03818958835 [Google Scholar] [CrossRef] [PubMed]
[16]. Hanefeld M, Koehler C, Schaper F, Fuecker K, Henkel E, Temelkova-Kurtschiev T, Postprandial plasma glucose is an independent risk factor for increased carotid intima-media thickness in non-diabetic individualsAtherosclerosis 1999 144:229-35.10.1016/S0021-9150(99)00059-3 [Google Scholar] [CrossRef]
[17]. Ketema EB, Kibret KT, Correlation of fasting and postprandial plasma glucose [17]with HbA1c in assessing glycaemic control; systematic review and meta-analysisArch Public Health 2015 73(1):4310.1186/s13690-015-0088-626413295 [Google Scholar] [CrossRef] [PubMed]
[18]. Shimizu H, Uehara Y, Okada S, Mori M, Contribution of fasting and postprandial hyperglycaemia to heamoglobin A1c in Insulin treated diabetic Japanese patientsEndocr J 2008 55(4):753-56.10.1507/endocrj.K07E-14218497454 [Google Scholar] [CrossRef] [PubMed]
[19]. Monnier L, Lapinski H, Colette C, Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycaemia of type 2 diabetic patientsDiabetes Care 2003 26(3):881-85.10.2337/diacare.26.3.88112610053 [Google Scholar] [CrossRef] [PubMed]
[20]. Bonora E, Calcaterra F, Lombardi S, Bonfante N, Formentini G, Bonadonna RC, Plasma glucose levels throughout the day and HbA1c interrelationships in type 2 diabetesDiabetes Care 2001 24(12):2023-29.10.2337/diacare.24.12.202311723077 [Google Scholar] [CrossRef] [PubMed]
[21]. Holman RR, Farmer AJ, Davies MJ, Levy JC, Darbyshire JL, Keenan JF, Three-year efficacy of complex insulin regimens in type 2 diabetesN Engl J Med 2009 361(18):1736-47.10.1056/NEJMoa090547919850703 [Google Scholar] [CrossRef] [PubMed]
[22]. Tkac I, Cardiovascular Importance of hyperglycaemia and hypoglycaemiaDiabetes Care 2013 36(2):267-71.10.2337/dcS13-204423882057 [Google Scholar] [CrossRef] [PubMed]
[23]. de Vegt F, Dekker JM, Ruhe HG, Nijpels CD, Bouter LM, Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn StudyDiabetologia 1999 42:926-31.10.1007/s00125005124910491751 [Google Scholar] [CrossRef] [PubMed]
[24]. Donahue RP, Abbott RD, Reed DM, Yano K, Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry: Honolulu Heart ProgramDiabetes 1987 36(1):689-92.10.2337/diab.36.6.6893569669 [Google Scholar] [CrossRef] [PubMed]
[25]. Lowe LP, Liu K, Greenland P, Metzger BE, Dyer AR, Stamler J, Diabetes, asymptomatic hyperglycaemia, and 22-year mortality in black and white men: the Chicago Heart Association Detection Project in Industry studyDiabetes Care 1997 20(2):163-69.10.2337/diacare.20.2.1639118765 [Google Scholar] [CrossRef] [PubMed]
[26]. Hanefeld M, Fischer S, Julius U, Schulze J, Schwanebeck U, Schmechel H, Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-upDiabetologia 1996 39(12):1577-83.10.1007/s0012500506178960845 [Google Scholar] [CrossRef] [PubMed]
[27]. DECODE study group. Glucose tolerance and mortality: comparison of WHO and American Diabetic Association diagnositc criteriaLancet 1999 354(9179):617-21.10.1016/S0140-6736(98)12131-1 [Google Scholar] [CrossRef]
[28]. Coutinho M, Gerstein HC, Wang Y, Yusuf S, The relationship between glucose and incident cardiovascular events: a metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 yearsDiabetes Care 1999 22(2):233-40.10.2337/diacare.22.2.23310333939 [Google Scholar] [CrossRef] [PubMed]
[29]. Hurel SJ, Mohan V, Clinical decision making: managing postprandial [29]hyperglycaemiaJ Assoc Physicians India 2006 54:871-76. [Google Scholar]
[30]. Van Gaal L, De Leeuw I, Rationale and options for combination therapy in the [30]treatment of Type 2 diabetesDiabetologia 2003 46(Suppl 1):M44-50.10.1007/s00125-002-0936-012652358 [Google Scholar] [CrossRef] [PubMed]
[31]. Jindal R, Gupta N, Siddiqui MA, Wangnoo SK, Postprandial hyperglycaemiaJIACM 2013 14(3-4):242-46. [Google Scholar]
[32]. Meher D, Dutta D, Ghosh S, Mukhopadhyay P, Chowdhury S, Mukhopadhyay S, Effect of a mixed meal on plasma lipids, insulin resistance and systemic inflammation in non-obese Indian adults with normal glucose tolerance and treatment naéve type-2 diabetesDiabetes Res Clin Pract 2014 104(1):97-102.10.1016/j.diabres.2013.12.04724461623 [Google Scholar] [CrossRef] [PubMed]
[33]. Kaku K, Efficacy of voglibose in type 2 diabetesExpert Opin Pharmacother 2014 15(8):1181-90. 10.1517/14656566.2014.91895624798092 [Google Scholar] [CrossRef] [PubMed]
[34]. The Japan Diabetes Society. Evidence-based Practice Guideline for the Treatment for Diabetes in Japan 2013. Treatment with Glucose-Lowering Agents (Excluding Insulin). Available at: http://www.jds.or.jp/modules/en/index.php?content_id=44 [Accessed 11 December 2017] [Google Scholar]
[35]. Yamaguchi M, Saji T, Mita S, Kulmatycki K, He YL, Furihata K, Pharmacokinetic and pharmacodynamic interaction of vildagliptin and voglibose in Japanese patients with Type 2 diabetesInt J Clin Pharmacol Ther 2013 51(8):641-51.10.5414/CP20190223782587 [Google Scholar] [CrossRef] [PubMed]
[36]. Imamura A, Kusunoki M, Ueda S, Hayashi N, Imai Y, Impact of voglibose on the pharmacokinetics of dapagliflozin in japanese patients with type 2 diabetesDiabetes Ther 2013 4(1)(1):41-49.10.1007/s13300-012-0016-523307267 [Google Scholar] [CrossRef] [PubMed]
[37]. Lee MY, Choi DS, Lee MK, Lee HW, Park TS, Kim DM, Comparison of acarbose and voglibose in diabetes patients who are inadequately controlled with basal insulin treatment: randomized, parallel, open-label, active-controlled studyJ Korean Med Sci 2014 29(1):90-97.10.3346/jkms.2014.29.1.9024431911 [Google Scholar] [CrossRef] [PubMed]
[38]. Ismail D, Deshmukh S, Comparative study of effect of alpha glucosidase inhibitors-Miglitol, acarbose and voglibose on postprandial Hyperglycaemia and glycosylated hemoglobin in type-2 Diabetes MellitusInt J Pharm Bio Sci 2012 3(3):337-43. [Google Scholar]
[39]. Fujitani Y, Fujimoto S, Takahashi K, Satoh H, Hirose T, Hiyoshi T, Effects of linagliptin monotherapy compared with voglibose on postprandial blood glucose responses in Japanese patients with type 2 diabetes: Linagliptin Study of Effects on Postprandial blood glucose (L STEP)Diabetes Res Clin Pract 2016 121:146-56.10.1016/j.diabres.2016.09.01427710821 [Google Scholar] [CrossRef] [PubMed]
[40]. Seo C, Sakamoto M, Nishimura R, Tsujino D, Ando K, Morimoto A, Comparison of glycaemic variability in patients with type 2 diabetes given sitagliptin or voglibose: a continuous glucose monitoring based pilot studyDiabetes Technol Ther 2013 15(5):378-85.10.1089/dia.2012.026223634671 [Google Scholar] [CrossRef] [PubMed]
[41]. Vichayanrat A, Ploybutr S, Tunlakit M, Watanakejorn P, Efficacy and safety of voglibose in comparison with acarbose in type 2 diabetic patientsDiabetes Res Clin Pract 2002 55(2):99-103.10.1016/S0168-8227(01)00286-8 [Google Scholar] [CrossRef]
[42]. Woo JT, Kim YS, Choi YK, Kim JW, Yang IM, Kim SW, Lowering effect of voglibose, monotherapy on uncontrolled postprandial glucose in patients with NonInsulin Dependent Diabetes Mellitus (NIDDM) being treated with strict diet control: multicenter open studyJ Korean Diabetes Assoc 1998 22(3):419-28. [Google Scholar]
[43]. Iwamoto Y, N N, Tajima Kadowaki, Nonaka K, Taniguchi T, Nishii M, Efficacy and safety of sitagliptin monotherapy compared with voglibose in Japanese patients with type 2 diabetes: a randomized, double blind trialDiabetes Obes Metab. 2010 12(7):613-22.10.1111/j.1463-1326.2010.01197.x20590736 [Google Scholar] [CrossRef] [PubMed]
[44]. Ohta A, Ohshige T, Sakai K, Nakamura T, Tenjin A, Tsukiyama S, Comparison of the hypoglycaemic effect of sitagliptin versus the combination of mitiglinide and voglibose in drugnaéve Japanese patients with type 2 diabetesExpert Opin Pharmacother 2013 14(17):2315-22.10.1517/14656566.2013.84255424079645 [Google Scholar] [CrossRef] [PubMed]
[45]. Katsuno T, Watanabe N, Nagai E, Okazaki K, Yokoyama A, Hamaguchi T, Comparison of efficacy of concomitant administration of mitiglinide with voglibose and double dose of mitiglinide in patients with type 2 diabetes mellitus: Mitiglinide treatment with vogliboseJ Diabetes Investig 2011 2(3):204-09.10.1111/j.2040-1124.2010.00082.x24843485 [Google Scholar] [CrossRef] [PubMed]
[46]. Inoue M, Tighter control of postprandial hyperglycaemia with mitiglinide/ voglibose fixed dose combination in Japanese patients with type 2 diabete mellitusExpert Opin Pharmacother 2012 13(16):22576810.1517/14656566.2012.72661422994875 [Google Scholar] [CrossRef] [PubMed]
[47]. Saito N, Sakai H, Suzuki S, Sekihara H, Yajima Y, Effect of an alpha glucosidase inhibitor (voglibose), in combination with sulphonylureas, on glycaemic control in type 2 diabetes patientsJ Int Med Res 1998 26(5):219-32.10.1177/0300060598026005019924706 [Google Scholar] [CrossRef] [PubMed]
[48]. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trialLancet 2002 359(9323):2072-77.10.1016/S0140-6736(02)08905-5 [Google Scholar] [CrossRef]
[49]. Nakamura K, Oe H, Kihara H, Shimada K, Fukuda S, Watanabe K, DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE studyCardiovasc Diabetol 2014 13:01-10.10.1186/s12933-014-0110-225074318 [Google Scholar] [CrossRef] [PubMed]
[50]. Yamasaki Y, Katakami N, Hayaishi-Okano R, Matsuhisa M, Kajimoto Y, Kosugi K, Alpha-Glucosidase inhibitor reduces the progression of carotid intimamedia thicknessDiabetes Res Clin Pract 2005 67(1):204-10.10.1016/j.diabres.2004.07.01215713352 [Google Scholar] [CrossRef] [PubMed]
[51]. Satoh N, Shimatsu A, Yamada K, Aizawa-Abe M, Suganami T, Kuzuya H, An alpha-glucosidase inhibitor, voglibose, reduces oxidative stress markers and soluble intercellular adhesion molecule 1 in obese type 2 diabetic patientsMetabolism 2006 55(6):786-93.10.1016/j.metabol.2006.01.01616713439 [Google Scholar] [CrossRef] [PubMed]
[52]. Fujitaka K, Otani H, Jo F, Jo H, Nomura E, Iwasaki M, Comparison of metabolic profile and adiponectin level with pioglitazone versus voglibose in patients with type-2 diabetes mellitus associated with metabolic syndromeEndocr J 2011 58(6):425-32.10.1507/endocrj.K10E-32721498915 [Google Scholar] [CrossRef] [PubMed]
[53]. Jackson CA, Yudkin JS, Forrest RD, A comparison of the relationships of the glucose tolerance test and the glycated hemoglobin assay with diabetic vascular disease in the community: the Islington Diabetes SurveyDiabetes Res Clin Pract 1992 17(2):111-23.10.1016/0168-8227(92)90156-L [Google Scholar] [CrossRef]
[54]. Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K, Voglibose for prevention of type 2 diabetes mellitus: a randomised, double blind trial in Japanese individuals with impaired glucose toleranceLancet 2009 373(9675):1607-14.10.1016/S0140-6736(09)60222-1 [Google Scholar] [CrossRef]
[55]. Dewda PR, Agrawal S, Role of voglibose in the treatment of prediabetes in Indian population: a cross-speciality surveyJ Clin Diagn Res 2013 7(10):2258-60.10.7860/JCDR/2013/5787.348624298491 [Google Scholar] [CrossRef] [PubMed]