Introduction
Periodontitis and diabetes mellitus are chronic inflammatory diseases with common risk factors. Periodontitis is initiated when the host response is unable to ward off the attack by putative microorganisms which thrive in the plaque biofilm.
As the disease progresses, the host response is exaggerated, wherein there is an increased production of inflammatory cytokines, Matrix Metalloproteinases (MMPs), Reactive Oxygen Species (ROS) which in turn cause deleterious effects on the periodontium and alveolar bone [1,2]. Diabetes mellitus, a globally rampant disease, occurs when the body does not produce enough insulin to maintain optimal levels of glucose in the blood, resulting in hyperglycemia. Hyperglycemia initiates oxidative stress and also stimulates the Receptor Activator of Nuclear factor Kappa B Ligand (RANKL), Osteoprotegerin (OPG) pathway to cause immune dysfunction, cellular stress and cytokine imbalance. Advanced glycation end products which are formed due to the increased blood glucose levels aggravate the condition, resulting in enhanced tissue destruction and periodontitis [3,4]. Resistin is an adipokine whose levels are elevated in patients afflicted with diabetes [5,6,7]. Periodontitis can also initiate diabetes as the microorganisms dampen the immune response of the host, causing insulin resistance and thus diabetes [8]. Both the diseases stimulate the release of ROS which along with the MMPs and cytokines cause connective tissue and bone loss. SRP (NSPT) as a therapeutic option has been reported in many studies to improve the HbA1c levels and thus the diabetic status of the individual gets better [9-11]. Antioxidant therapy directed against the ROS has also been reported to improve the disease outcomes of both the diseases [12,13]. The aim of the present study was to assess the beneficial effects of NSPT and ALA administration on HbA1c and resistin levels in patients with both diabetes and periodontitis.
Materials and Methods
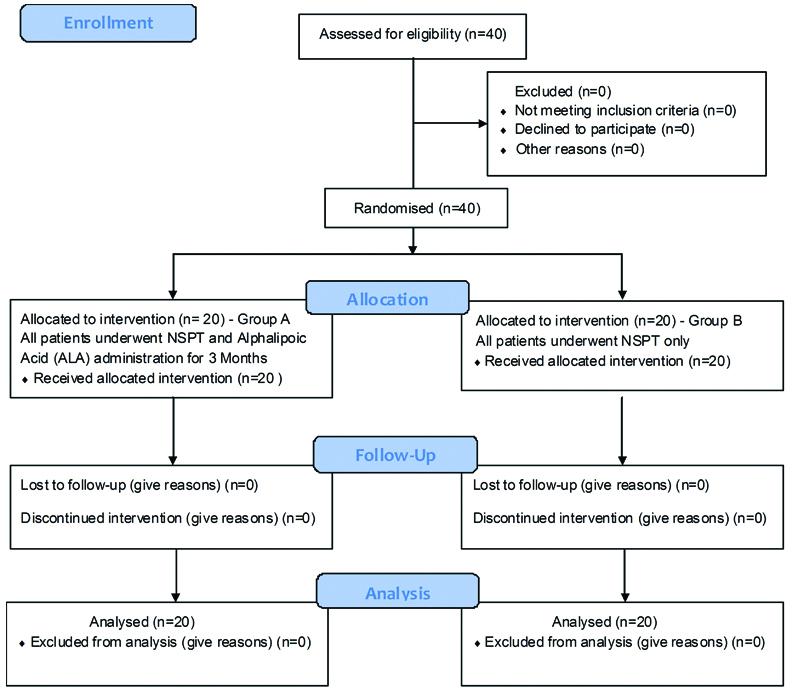
This was a randomised interventional single blinded clinical trial which was carried out in Department of Periodontics of Panineeya Mahavidyalaya Institute of Dental Sciences and Research Centre in collaboration with Mohan’s Diabetic Centre, Domalguda, Hyderabad, Telangana, India. The patients were selected from the outpatient ward of Mohan’s Diabetic centre, and included both the sexes. The guidelines of Helsinki declaration of 1975 as revised in 2000, were strictly followed while conducting the study. This study was approved by the Institutional Review Board and registered in clinical trials registry (NCT 02775266). A written informed consent form was signed by each patient before the start of the study. To get a mean difference of 8.20 in serum resistin levels between the test and control group with a standard deviation of 8.7, with power being 80% and level of significance 5%, the required sample size for each group was calculated to be 20 [6]. This study included 40 patients with uncontrolled diabetes mellitus (HbA1c levels >6.5 upto 10%) and generalised chronic periodontitis, aged between 35-60 years, with a mean age of 50.3 years, who were equally divided into group A (test) and group B (control) [Table/Fig-1].
CONSORT 2010 flow diagram.
Inclusion Criteria
Patients recently diagnosed (<1 month) with Type 2 diabetes with HbA1c levels ≥6.5%. As per ADA (American Diabetes Association) criteria and taking metformin 500 mg/day by oral route, and chronic periodontitis as per American Academy of Periodontology (AAP) guidelines (which included presence of minimum of 15 natural teeth and at least four teeth with one or more sites with Probing Depth (PD) ≥5 mm, CAL ≥4 mm and Bleeding On Probing (BOP)) were included in the study [14,15].
Exclusion Criteria
Patients with systemic illness other than diabetes, smokers, pregnant and lactating women, patients who had undergone SRP and those who were on systemic antibiotics for the past three months were excluded from the study.
Selection Criteria: All the patients meeting the selection criteria were consecutively enrolled from December 2015 to February 2016. A total of 20 patients in group A (test group) underwent SRP after which ALA 600 mg was administered systemically thrice a day for three months [12]. In group B (control group) 20 patients underwent SRP only. The eligible samples were screened and randomly assigned by lottery method by investigator KRR into group A and group B. The treatment was performed by investigator SK who was blinded to the randomisation process.
Clinical Parameters
All patients were subjected to a periodontal clinical examination performed in six sites per tooth using William’s periodontal probe. The clinical parameters assessed were the GI, PPD and CAL [16-18].
Biochemical Parameters
Collection of Blood Sample and Serum Separation
Random blood samples were collected by venepuncture of anticubital vein. Around 2 mL of blood was collected. About 1 mL was placed in each test tube and 1 mL of whole blood was used for HbA1c estimation. Also, 10 minutes after collection the other test tube containing 1 mL blood was subjected to centrifugation at 3000 rpm for 10 minutes. The supernatant straw colored fluid (serum) was separated into two storage vials for serum resistin.
Assessment of Serum Resistin
It was done at baseline and three months after NSPT by commercial kit (R and D systems a bio-techne brand USA) [19]. The assay employs the quantitative sandwich enzyme immunoassay technique [Table/Fig-2].
Human resistin ELISA kit.

Assessment of Glycosylated Haemoglobin
Assessment of HbA1c was done at baseline and three months by using spectrophotometric analysis (Erba chem 5 plus v2, Transasia Biomedicals Limited, Mumbai) [Table/Fig-3] [20].
Spectrophotometer for assessment of HbA1c.

Primary and Secondary Outcome Measures
The Primary outcome measures assessed were the serum resistin and HbA1c levels and the secondary parameters assessed were the GI, PPD and CAL.
Statistical Analysis
Data was analysed by SPSS version 22.0. Data was summarised by mean±Standard Deviation (SD) for continuous data and median±IQR (InterQuartile Range) for score data. Data was summarised by Percentages for categorical data. The comparison between baseline and three months was done by paired t-test for continuous data and Wilcoxon signed rank test for score data. The comparison between two groups was done by unpaired t test for continuous data and Mann Whitney U test for score data. The association between two groups was done by chi-square test/Fishers exact test for categorical data. All p-values less than 0.05 were considered as statistically significant.
Results
The intragroup comparison in group A (test group) showed that there was a statistically significant difference pertaining to all the clinical (GI, PPD, CAL) and biochemical parameters (HbA1c and serum resistin) from baseline to three months with p<0.001 [Table/Fig-4]. In group B (control group) also, there was a statistically significant difference pertaining to all the clinical (GI, PPD, CAL) and biochemical parameters (HbA1c and serum resistin) from baseline to three months p<0.001 [Table/Fig-5].
Intragroup comparison in group A (test group) of clinical and biochemical parameters.
| Parameters* | Assessment | n‡ | Range | Mean | SD† | p-value |
|---|
| GI | Baseline | 20 | 2.2-3.0 | 2.6 | 0.3 | <0.001 |
| 3 months | 20 | 0.6-1.3 | 1.0 | 0.2 |
| PD | Baseline | 20 | 7-9 | 8.1 | 0.6 | <0.001 |
| 3 months | 20 | 3-4 | 3.8 | 0.4 |
| CAL | Baseline | 20 | 5-7 | 6.1 | 0.6 | <0.001 |
| 3 months | 20 | 1-2 | 1.7 | 0.5 |
| HbA1c% | Baseline | 20 | 9.3-10.7 | 9.9 | 0.3 | <0.001 |
| 3 months | 20 | 6-7 | 6.3 | 0.3 |
| Serum resistin (ng/mL) | Baseline | 20 | 20.3-36.7 | 27.2 | 5.4 | <0.001 |
| 3 months | 20 | 8.5-29 | 16.7 | 8.0 |
*Clinical and biochemical parameters: GI: Gingival index; PD: Probing depth; CAL: Clinical attachment levels; HbA1c%: Glycated haemoglobin percentage and serum resistin levels.
†SD: Standard deviation
‡n: Number of patients
Paired t-test was done for GI, HbA1c%, serum resistin (ng/mL) and Wilcoxon signed rank test was used for PD and CAL.
Intragroup comparison in group B (control group) of clinical and biochemical parameters.
| Parameters* | Assessment | n‡ | Range | Mean | SD† | p-value |
|---|
| GI | Baseline | 20 | 2.2-3.0 | 2.6 | 0.2 | <0.001 |
| 3 months | 20 | 0.8-1.9 | 1.3 | 0.3 |
| PD | Baseline | 20 | 6-10 | 8.0 | 1.3 | <0.001 |
| 3 months | 20 | 3-6 | 4.6 | 0.8 |
| CAL | Baseline | 20 | 4-8 | 6.0 | 1.2 | <0.001 |
| 3 months | 20 | 1-4 | 2.6 | 0.8 |
| HbA1c % | Baseline | 20 | 7.1-10.3 | 8.6 | 1.1 | <0.001 |
| 3 months | 20 | 6.3-9.5 | 7.4 | 0.7 |
| Serum resistin (ng/mL) | Baseline | 20 | 22-35.9 | 27.2 | 4.6 | <0.001 |
| 3 months | 20 | 20.1-32.5 | 24.9 | 3.7 |
*Clinical and biochemical parameters: GI: Gingival index; PD: Probing depth; CAL: Clinical attachment levels; HbA1c%: Glycated haemoglobin percentage and serum resistin levels.
†SD: Standard deviation
‡n-Number of patients
Paired t-test was done for GI, HbA1c%, serum resistin (ng/mL) and Wilcoxon signed rank test was used for PD and CAL.
However, when an intergroup comparison was made it was observed that all the clinical and biochemical parameters showed better and statistically significant results in group A (systemic ALA+NSPT) when compared to group B (NSPT only) [Table/Fig-6].
Intergroup comparison of clinical and biochemical parameters at three months.
| Parameters* | Groups | n‡ | Range | Mean | SD† | p-value |
|---|
| GI | Control 3 months | 20 | 0.8-1.9 | 1.3 | 0.3 | 0.002 |
| Test 3 months | 20 | 0.6-1.3 | 1.0 | 0.2 |
| PD | Control 3 months | 20 | 3-6 | 4.6 | 0.8 | 0.0005 |
| Test 3 months | 20 | 3-4 | 3.8 | 0.4 |
| CAL | Control 3 months | 20 | 1-4 | 2.6 | 0.8 | <0.001 |
| Test 3 months | 20 | 1-2 | 1.7 | 0.5 |
| HbA1c% | Control 3 months | 20 | 6.3-9.5 | 7.4 | 0.7 | <0.001 |
| Test 3 months | 20 | 6-7 | 6.3 | 0.3 |
| Serum resistin (ng/mL) | Control 3 months | 20 | 20.1-32.5 | 24.9 | 3.7 | 0.0002 |
| Test 3 months | 20 | 8.5-29 | 16.7 | 8.0 |
*Clinical and biochemical parameters: GI: Gingival index; PD: Probing depth; CAL: Clinical attachment levels; HbA1c%: Glycated haemoglobin percentage and serum resistin levels
†SD: Standard deviation
‡n: Number of patients
Unpaired t-test was done for GI, HbA1c%, serum resistin (ng/mL) and Mann Whitney U test for PD and CAL.
Discussion
Periodontitis is a complex polymicrobial disease in which inflammation in the periodontal tissues is stimulated by the long term presence of the subgingival plaque. The inflammatory response is characterised by dysregulated secretion of host derived mediators of inflammation and tissue breakdown. The most extensively studied include IL-1β, IL-6, Prostaglandin E2 (PGE2), TNF-α, RANKL, and the MMP’s particularly MMP-8, MMP-9 and MMP-13, as well as T cell regulatory cytokines (e.g., IL-12, IL-18) and the chemokines [21]. The etiology of type 2 diabetes appears to be multifactorial. A genetic component contributes to individual susceptibility for the development of type 2 diabetes. Metabolic syndrome, the characteristics of which are impaired glucose tolerance, obesity, hypertension and dyslipidemia, is associated with an increased risk for the development of diabetes by a factor of 2.99 and may be considered a ‘pre diabetic’ state. Type 2 diabetes and the ‘pre diabetic’ metabolic syndrome are associated with obesity, physical inactivity and a high glucose, high fat, low fibre diet. Both the diseases are interlinked and one disease may lead to the other [22]. Many studies have given conclusive evidence about diabetes worsening periodontitis and vice versa [23-26].
In a study conducted by Alshehri FA, and Javed F, 50 pre diabetic patients were enrolled and divided equally into two groups. The test group (25 patients) received SRP along with oral doxycycline 100 mg, whereas the control group (25 patients) received SRP only. In both the groups the clinical parameters and fasting blood glucose levels were assessed at baseline and after six months. It was observed that both groups showed an improvement in the clinical parameters after six months, however there was no improvement in the fasting blood glucose levels after six months in both the groups [27].
In this study also there was a statistically significant difference in all the clinical parameters in both the groups. However the results were more significant in group A (SRP+ALA 600 mg thrice daily for three months) when compared to group B (SRP only).
In a study to examine if HbA1c levels are elevated in patients with periodontitis, 70 subjects with periodontitis and 70 healthy controls were enrolled. It was observed that the HbA1c levels in patients with periodontitis were significantly higher than the control group. The resultant hyperglycaemic state may increase the risk for diabetes [28]. There are conflicting reports related to the improvement in HbA1c levels in diabetic patients after SRP. In a meta analysis which was conducted in 2005, gathering reports from ten interventional studies, done to examine the effects of periodontal treatment on HbA1c levels in diabetic patients, it was concluded that HbA1c levels decreased by 0.38% for all the studies, which was not statistically significant [29]. However, some of the studies conducted later concluded that there was an improvement in the HbA1c levels in the test group (diabetic patients with periodontitis who underwent SRP) [Table/Fig-7] [30-34].
Studies conducted to assess the effect of scaling and root planing on HbA1c levels.
| Author (Year) | Country | Sample size | Inclusion criteria for diabetic patients-HbA1c% | Inclusion criteria for chronic periodontitis patients | Intervention | Duration of study | Changes in HbA1c levels after intervention |
|---|
| Koromantzos PA et al., (2011) [30] | Greece | 60 Test=30 Control=30 | Type 2 DM HbA1C levels (7%-10%) Initial HbA1c: T=7.0-9.9 C=7.0-10.2 | Having at least 16 teeth present with at least eight sites (PPD) >6 mm and four sites with CAL >5 mm, distributed in at least two different quadrants. | T=SRP+OHI C=Delayed treatment | 6 months | HbA1c changes within the 1st 3months T=0.73±0.66 C=0.18±0.59 HbA1c changes after 6th month T=0.72±0.93 C=0.13±0.46 |
| Engebretson SP et al., (2013) [31] | USA | 50 Test=25 Control=25 | Type 2 DM HbA1c level (7.0%-9.0%) Initial HbA1c: T=7.84 (0.65) C=7.78 (0.60) | A minimum of 16 natural teeth. CAL/PD >5 mm in two or more quadrants of the mouth. No periodontal treatment in the prior six months. | T=SRP+OHI C=OHI | 6 months | Mean change of HbA1c (95% CI) at 3rd month T=0.14 (0.02-0.27) C=0.11 (-0.02 to 0.24) Mean change of HbA1c (95% CI) at the end of 6th month T=0.15 (-0.01 to 0.30) C=0.09 (-0.06 to 0.25) |
| Kanduluru A and Naganandini S (2014) [32] | India | 70 Test=35 Control=35 | Type 2 DM HbA1c level Initial HbA1c: T=8.49±1.50 C=8.04±0.70 | Pocket Depth [PD] 4-6 mm involving >30% sites Generalised moderate periodontitis | T=SRP+OHI C=OHI | 3 months | HbA1c Changes after 3rd month T=8.47±0.89 C=8.27±0.63 |
| Gay IC et al., (2014) [33] | USA | 126 Test=66 Control=60 | Type 2 DM HbA1c level (4.0-15.0%) Initial HbA1c: T=9.0±2.3 C=8.4±2.02. nonsmoker | No systemic antibiotic therapy within six months of recruitment. The presence of localised or generalised severe chronic periodontitis | T=SRP+OHI C=OHI | 4 months | HbA1c Changes after 4th month T=8.4±1.9 C=8.1±1.8 |
| Kaur PK et al., (2015) [34] | India | 100 Test=50 Control=50 | Type 2DM Initial HbA1c: T=8.17±2.49 C=7.87±2.562 | Presence of ≥12 teeth, clinical diagnosis of moderate and severe periodontitis. Moderate periodontitis: ≥2 interproximal sites, not on the same tooth, with an attachment loss ≥4 mm, or PD ≥5 mm. Severe periodontitis: ≥2 interproximal sites, not on the same tooth, with an attachment loss ≥6 mm, and one or probing depth ≥5 mm | T=OHI+SRP C=No treatment | 3,6 months | HbA1c changes in the end of 3rd month. T=7.49±1.83 C=7.96±2.65 HbA1c changes in the end of 6th month. T=7.29±1.61 C=8.06±2.72 |
T: test; C: Control; OHI: Oral hygiene instructions; DM: Diabetes mellitus
In this study it was observed that the HbA1c levels reduced in both the groups after SRP, however, highly significant results were obtained in group A (9.9 to 6.3%) (p<0.001) when compared to group B (8.6 to 7.4%). Oxidative stress (which is an imbalance between reactive species and antioxidants produced by the body) pays a pivotal role in the etiopathogenesis of most diseases and diabetes is no exception. The hyperglycaemic state induces the production of free radicals and impairs the endogenous antioxidant defence system of the host [35]. Resistin is an adipokine whose levels are elevated in inflammatory conditions. This enzyme causes insulin resistance and is often associated with diabetes and obesity [36,37]. A study was carried out to investigate the relationship between periodontal conditions and serum levels of resistin and adiponectin in elderly Japanese. It was demonstrated that both circulating resistin levels and total leukocytes and neutrophil counts were significantly elevated in subjects with periodontitis when compared with controls [37].
A total of 40 subjects were enrolled in another study of whom 20 were healthy (control group) and 20 had chronic periodontitis (test group). Periodontal parameters Plaque Index (PI), GI, Bleeding Index (BI), PPD, CAL together with serum resistin levels were assessed at baseline and between 6-8 weeks following NSPT for subjects. Clinical parameters and serum resistin levels were assessed both at baseline and 6-8 weeks after NSPT in samples in the test group and only at baseline in the control group. There was a statistically significant difference in the clinical parameters at baseline between the groups and significant improvement in the test group after SRP. Though, the serum resistin levels were higher in the test group when compared to the control group at baseline, the values were not statistically significant. Moreover, 6-8 weeks after scaling also, serum resistin levels decreased in the test group and no significant difference was found in their levels between baseline and postintervention [38].
The present study showed elevated resistin levels at baseline in both the test and control groups; however, it was observed that there was a reduction in resistin levels in group A (27.2 to 16.7ng/mL) as well as in group B (27.2 to 24.9 ng/mL) three months after SRP. However, the reduction in resistin levels were highly significant in group A (p=0.0002) when compared to group B.
ALA is a powerful antioxidant that is able to scavenge a number of free radicals, such as superoxide radicals, singlet oxygen, hydroxyl radicals and hypochlorous acid. It occurs with a chelate of Fe2+ and Fe3+ in both hydrophilic and lipophilic environments. The ALA present in nature is always covalently bonded and not readily available from dietary sources. It is able to cross the blood brain barrier without any serious side effects and has been shown to improve glucose metabolism in diabetic patients [39].
Drug Adherence/Safety: No adverse effects were reported in this study with ALA administration.
Conclusion
ALA could have been administered for a longer period of time. The sample size could have been larger to better validate the results.
Many studies have already been done on the beneficial role of ALA supplementation in diabetic patients; however, the effect of ALA administration on patients with both diabetes and chronic periodontitis has not been assessed. Thus, in future many more such studies can be done to throw light on the benefits of ALA supplementation.
Conclusion
This study shows that ALA as an adjuvant after SRP has a beneficial role both in combating oxidative stress induced tissue destruction and restoring glycaemic control in patients with chronic periodontitis and type 2 diabetes mellitus, however many more studies have to be done to assert its positive effects on the periodontium.
*Clinical and biochemical parameters: GI: Gingival index; PD: Probing depth; CAL: Clinical attachment levels; HbA1c%: Glycated haemoglobin percentage and serum resistin levels.†SD: Standard deviation‡n: Number of patientsPaired t-test was done for GI, HbA1c%, serum resistin (ng/mL) and Wilcoxon signed rank test was used for PD and CAL.
*Clinical and biochemical parameters: GI: Gingival index; PD: Probing depth; CAL: Clinical attachment levels; HbA1c%: Glycated haemoglobin percentage and serum resistin levels.†SD: Standard deviation‡n-Number of patientsPaired t-test was done for GI, HbA1c%, serum resistin (ng/mL) and Wilcoxon signed rank test was used for PD and CAL.
*Clinical and biochemical parameters: GI: Gingival index; PD: Probing depth; CAL: Clinical attachment levels; HbA1c%: Glycated haemoglobin percentage and serum resistin levels†SD: Standard deviation‡n: Number of patientsUnpaired t-test was done for GI, HbA1c%, serum resistin (ng/mL) and Mann Whitney U test for PD and CAL.
T: test; C: Control; OHI: Oral hygiene instructions; DM: Diabetes mellitus
[1]. Loe H, Periodontal disease-The sixth complication of diabetes mellitus: diabetes careTeratology 1993 16(1):329-34.10.2337/diacare.16.1.3298422804 [Google Scholar] [CrossRef] [PubMed]
[2]. Kesic L, Milasin J, Igic M, Obradovic R, Microbial etiology of periodontal disease mini reviewMed Biol 2008 15:1-6. [Google Scholar]
[3]. Abdulfatai B. Olokoba, Olusegun A. Obateru, Lateefat B. Olokoba, Type 2 diabetes mellitus: a review of current trendsOman Med J 2012 27(4):269-73.10.5001/omj.2012.6823071876 [Google Scholar] [CrossRef] [PubMed]
[4]. Blair M, Diabetes mellitus reviewUrol Nurs 2016 36(1):27-36.10.1177/0956474816636829 [Google Scholar] [CrossRef]
[5]. Patel SP, Raju PA, Resistin in serum and gingival crevicular fluid as a marker of periodontal inflammation and its correlation with single-nucleotide polymorphism in human resistin gene at -420Contemp Clin Dent 2013 4(2):192-97.10.4103/0976-237X.11487824015008 [Google Scholar] [CrossRef] [PubMed]
[6]. Gokhale NH, Acharya AB, Patil VS, Trivedi DJ, Setty S, Thakur SL, Resistin levels in gingival crevicular fluid of patients with chronic periodontitis and type 2 diabetes mellitusJ Periodontol 2014 85(4):610-17.10.1902/jop.2013.13009223805816 [Google Scholar] [CrossRef] [PubMed]
[7]. Mittal M, Hassan B, Desai K, Duseja S, Kumar S, Reddy SG, GCF resistin as a novel marker in patients with chronic periodontitis and rheumatoid arthritisJ Clin Diagn Res 2015 9(4):ZC62-ZC64.10.7860/JCDR/2015/12327.584926023646 [Google Scholar] [CrossRef] [PubMed]
[8]. Chapple IL, Genco R, Working group 2 of the joint EFP/AAP workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP workshop on periodontitis and systemic diseasesJ Periodontol 2013 84(4):106-12.10.1902/jop.2013.134001123631572 [Google Scholar] [CrossRef] [PubMed]
[9]. Stewart JE, Wager KA, Friedlander AH, Zadeh HH, The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitusJ Clin Periodontol 2001 28:306-10.10.1034/j.1600-051x.2001.028004306.x11314885 [Google Scholar] [CrossRef] [PubMed]
[10]. Rodrigues DC, Taba MJ, Novaes AB, Souza SL, Grisi MF, Effect of non-surgical periodontal therapy on glycemic control in patients with type 2 diabetes mellitusJ Periodontol 2003 74:1361-67.10.1902/jop.2003.74.9.136114584871 [Google Scholar] [CrossRef] [PubMed]
[11]. Teeuw WJ, Gerdes VEA, Loos BG, Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysisDiabetes Care 2010 33:421-27.10.2337/dc09-137820103557 [Google Scholar] [CrossRef] [PubMed]
[12]. Golbidi S, Badran M, Laher I, Diabetes and alphalipoic acidFront Pharmacol 2011 17:2-69.10.3389/fphar.2011.0006922125537 [Google Scholar] [CrossRef] [PubMed]
[13]. Lakhtin IuV, Comparative evaluation of short- and long-term treatment of periodontitis with alpha-lipoic acidGeorgian Med News 2013 (218):19-22. [Google Scholar]
[14]. Burant CF, Young LA, Eds. Alexandria VA. American Diabetes AssociationMedical Management of Type 2 Diabetes 1974 7th Edition [Google Scholar]
[15]. Machtei EE, Christersson LA, Grossi SG, Dunford R, Zambon JJ, Genco RJ, Clinical criteria for the definition of “established periodontitis.J Periodontol 1992 63(3):206-14.10.1902/jop.1992.63.3.2061593413 [Google Scholar] [CrossRef] [PubMed]
[16]. Lğe H, The gingival index, the plaque index and the retention index systemsJ Periodontol 1967 38(6):610-16.10.1902/jop.1967.38.6.6105237684 [Google Scholar] [CrossRef] [PubMed]
[17]. Listgarten MA, Mao R, Robinson PJ, Periodontal probing and the relationship of the probe tip to periodontal tissuesJ Periodontol 1976 47(9):511-13.10.1902/jop.1976.47.9.5111067404 [Google Scholar] [CrossRef] [PubMed]
[18]. Pihlstrom BL, Measurement of attachment level in clinical trials: probing methodsJ Periodontol 1992 63:1072-107.10.1902/jop.1992.63.12s.1072 [Google Scholar] [CrossRef]
[19]. Human Resistin Quantikine ELISA Kit DR SNOO: R&D systems. Available from: https://www.rndsystems.com/products/human-resistin-quantikine-elisa-kit_drsn0 [Google Scholar]
[20]. Laboratory procedure manual CDC. Available from: https://www.cdc.gov/nchs/data/nhanes/nhanes_07.../ghb_e_met_tosoh_22_plus.pdf [Google Scholar]
[21]. Preshaw PM, Taylor JJ, How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis?J Clin Periodontol 2011 38(11):60-84.10.1111/j.1600-051X.2010.01671.x21323705 [Google Scholar] [CrossRef] [PubMed]
[22]. Taylor JJ, Preshaw PM, Lalla E, A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetesJ Clin Periodontol 2013 40(14):12910.1111/jcpe.1205923627323 [Google Scholar] [CrossRef] [PubMed]
[23]. Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Glycemic control and alveolar bone loss progression in type 2 diabetesAnn Periodontol 1998 3:30-39.10.1902/annals.1998.3.1.309722688 [Google Scholar] [CrossRef] [PubMed]
[24]. Chavarry NG M, Vettore MV, Sansone C, Sheiham A, The relationship between diabetes mellitus and destructive periodontal disease: a meta-analysisOral Health Prev Dent 2009 7:107-127. [Google Scholar]
[25]. Campus G, Salem A, Uzzau S, Baldoni E, Tonolo C, Diabetes and periodontal disease: a case-control studyJ Periodontol 2005 76(3):418-25.10.1902/jop.2005.76.3.41815857077 [Google Scholar] [CrossRef] [PubMed]
[26]. Nelson RG, Shlossman M, Budding LM, Pettitt DJ, Saad MF, Genco RJ, Periodontal disease and NIDDM in Pima IndiansDiabetes Care 1990 13:836-40.10.2337/diacare.13.8.8362209317 [Google Scholar] [CrossRef] [PubMed]
[27]. Alshehri FA, Javed F, Impact of scaling and root planing on clinical periodontal status and glycemic levels in prediabetic patientsInterv Med Appl Sci 2015 7(1):17-21.10.1556/IMAS.6.2014.00425838922 [Google Scholar] [CrossRef] [PubMed]
[28]. Rajan P, Nera M, Pavalura AK, Medandrao N, Kumar SC, Comparison of glycosylated hemoglobin (HbA1C) levels in patients with chronic periodontitis and healthy controlsDent Res J (Isfahan) 2013 10(3):389-93. [Google Scholar]
[29]. Janket SJ, Wightman A, Van Dyke TE, Jones JA, Does periodontal treatment improve glycemic control in diabetic patients? A meta-analysis of intervention studiesJ Dent Res 2005 84(12):1154-59.10.1177/15440591050840121216304446 [Google Scholar] [CrossRef] [PubMed]
[30]. Koromantzos PA, Makrilakis K, Dereka X, Katsilambros N, Vrotsos IA, Madianos PN, A randomized, controlled trial on the effect of non-surgical periodontal therapy in patients with type 2 diabetes. Part I: effect on periodontal status and glycaemic controlJ Clin Periodontol 2011 38(2):142-47.10.1111/j.1600-051X.2010.01652.x21114680 [Google Scholar] [CrossRef] [PubMed]
[31]. Engebretson SP, Hyman LG, Michalowicz BS, Schoenfeld ER, Gelato MC, Hou W, The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trialJAMA 2013 310(23):2523-32.10.1001/jama.2013.28243124346989 [Google Scholar] [CrossRef] [PubMed]
[32]. Kanduluru A, Naganandini S, Effect of nonsurgical periodontal treatment on clinical response and glycemic control in type 2 diabetic patients with periodontitis: controlled clinical trialJ Indian Assoc Public Health Dent 2014 12(4):261-67.10.4103/2319-5932.147643 [Google Scholar] [CrossRef]
[33]. Gay IC, Tran DT, Cavender AC, Weltman R, Chang J, Luckenbach E, The effect of periodontal therapy on glycaemic control in a Hispanic population with type 2 diabetes:a randomized controlled trialJ Clin Periodontol 2014 41(7):673-80.10.1111/jcpe.1226824797222 [Google Scholar] [CrossRef] [PubMed]
[34]. Kaur PK, Narula R, Rajput K, Sharma R, Tewari S, Periodontal and glycemic effects of nonsurgical periodontal therapy in patients with type 2 diabetes stratified by baseline HbA1cJ Oral Sci 2015 57(3):201-11.10.2334/josnusd.57.20126369484 [Google Scholar] [CrossRef] [PubMed]
[35]. Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J, The role of oxidative stress and antioxidants in diabetic complicationsSultan Qaboos Univ Med J 2012 12(1):5-18.10.12816/000308222375253 [Google Scholar] [CrossRef] [PubMed]
[36]. Berger A, Resistin: A new hormone that links obesity with type 2 diabetesBMJ 2001 322(7280):19310.1136/bmj.322.7280.21811159622 [Google Scholar] [CrossRef] [PubMed]
[37]. Furugen R, Hayashida H, Yamaguchi N, Yoshihara A, Ogawa H, Miyazaki H, The relationship between periodontal condition and serum levels of resistin and adiponectin in elderly JapaneseJ Periodontal Res 2008 43(1):556-62.10.1111/j.1600-0765.2008.01085.x18565135 [Google Scholar] [CrossRef] [PubMed]
[38]. Devanoorkar A, Dwarakanath CD, Gundanavar G, Kathariya R, Patil SR, Evaluation of serum resistin levels in periodontal health and disease and effects of non surgical periodontal therapy on its levelsDis Markers 2012 32:289-94.10.1155/2012/15341822848926 [Google Scholar] [CrossRef] [PubMed]
[39]. El-Hossary GG, El Karam SH, Efficacy of alpha deterioration of blood antioxidants and diabetic retinopathy in experimental animalsAust J Basic Appl Sci 2010 4:1 [Google Scholar]