Granular Cell Tumour of Breast-An Enigmatic Entity-Case Report with Emphasis on Role of Frozen Section
Pooja Jain1, Geetha Vasudevan2, Padmapriya Jaiprakash3, Stanley Mathew4
1 Junior Resident, Department of Pathology, Kasturba Medical College, Manipal, Karnataka, India.
2 Additional Professor, Department of Pathology, Kasturba Medical College, Manipal, Karnataka, India.
3 Associate Professor, Department of Pathology, Kasturba Medical College, Manipal, Karnataka, India.
4 Professor, Department of Surgery, Kasturba Medical College, Manipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Padmapriya Jaiprakash, Associate Professor, Department of Pathology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal-576104, Karnataka, India.
E-mail: padmapriya.j@gmail.com
Granular Cell Tumour (GCT) is a rare tumour involving the breast, pre- and intraoperative diagnosis of which plays an important role in deciding the treatment. Recognition of this usually benign tumour is important, since clinical (irregular and firm), radiological (ill defined or spiculated lesion without microcalcifications) and gross findings often mimic carcinoma. We report a case of a GCT of the breast mimicking carcinoma clinically and on mammography. The diagnosis was made by frozen section, confirmed by histological examination and immunohistochemistry (IHC). We emphasise the role of frozen section and intraoperative diagnosis in guiding treatment and preventing radical surgery. The clinico-radiological, histopathological and therapeutic aspects of this rare tumour, are also discussed.
Lump, Mammography, Ultrasonography
Case Report
A 41-year-old woman came with a chief complaint of lump in her right breast since four months. Local examination revealed a 2x2 cm hard and mobile, painless mass in retroareolar region of right breast. There were no enlarged nodes in the axilla. Ultrasonography (USG) showed a medial para-areolar hard solid lesion with lobulated to spiculated margins and loss of normal long to short axis ratio (L:S ratio) favouring aggressive features.
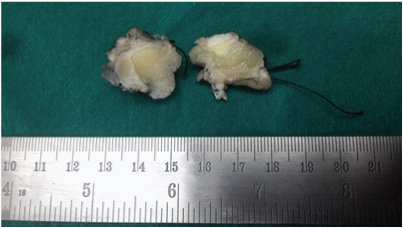
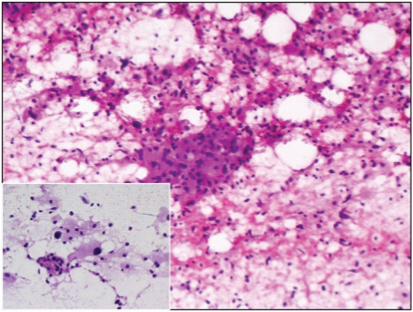
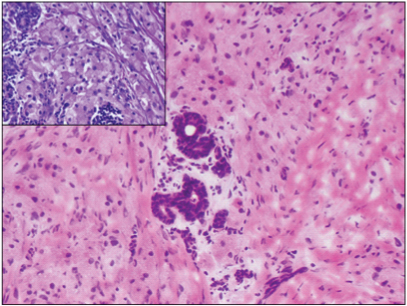
Fine Needle Aspiration Cytology (FNAC) done at another centre was reported as features suggestive of atypical ductal hyperplasia. In view of radiology and FNAC report, lumpectomy was performed and sent for intraoperative frozen section. Gross examination of the lumpectomy revealed an ill defined firm to hard nodular lesion, measuring 1.3x1.2x0.9 cm [Table/Fig-1]. Imprint smears from the lesion were cellular with occasional benign ductal epithelial cells surrounded by sheets of dyscohesive cells with abundant granular eosinophilic cytoplasm, suggestive of oncocytic or histiocytoid cells [Table/Fig-2]. Frozen section showed benign breast lobules surrounded by a tumour composed of cords, nests and trabecular arrangement of epithelioid cells with abundant granular eosinophilic cytoplasm and central pyknotic nuclei in a fibrocollagenous stroma suggestive of GCT [Table/Fig-3].
Fibrofatty mass with an ill defined firm to hard nodular grey white lesion.
Cellular smears showing sheets of cells with abundant granular eosinophilic cytoplasm, suggestive of oncocytic or histiocytoid cells with occasional benign ductal epithelial cells. Rapid H & E, 10X, 20X (inset).
Benign breast lobules surrounded by cords and nests of epithelioid tumour cells with abundant granular eosinophilic cytoplasm and central pyknotic nuclei. H & E, 20X (frozen section), 40X (paraffin section, inset).
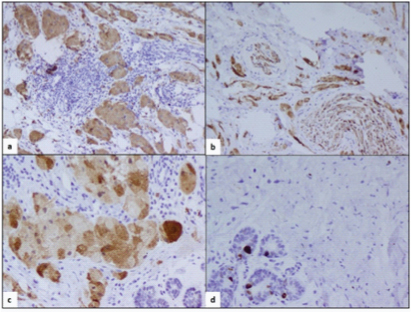
Paraffin section confirmed the frozen section findings [Table/Fig-3(inset)]. The IHC showed tumour cells stained with CD68 and S-100 protein [Table/Fig-4], the latter confirming the neural origin of the lesion. Ki67 labelling was low (1-2%) [Table/Fig-4]. On follow-up at second and sixth month, the patient was doing well after surgery, without any recurrence.
showing S-100 (a, b; 10X), CD68 (c; 20X) and Ki67 (d; 20X) positivity.
Discussion
The GCTs are neoplasms that usually pursue a benign clinical course. They were first recognised as a distinct clinical entity in 1926 [1]. They occur more frequently in females and over a wide age range (17-75 years) [2]. Most commonly found in head and neck region particularly the tongue, they are rarely seen at other sites [3]. GCT in breast is a rare occurrence, accounting for only 5-6% of all GCTs and in premenopausal black women [4]. Diagnostic problems arise due to the unusual location and also because clinical and radiological findings overlap with those of an infiltrating carcinoma. Further, it has to be distinguished from lesions with similar histological features.
On physical examination, GCT usually manifest as painless, firm and palpable mass, typically less than 3 cm. They may cause skin retraction, infiltration, or ulceration, all features mimicking breast cancers [5]. Radiological findings including ill defined or spiculated lesions indicate malignancy. However, microcalcifications are not common findings in GCTs. In contrast to most other breast tumours, which occur primarily in the upper outer quadrant, most breast GCTs occur in the upper inner quadrant, corresponding to the sensory distribution of the supraclavicular nerve. The current case however, was a para-areolar hard solid lesion with spiculated margins on USG.
Fine-needle aspiration biopsy and frozen section are helpful in identifying GCT [5]. Tumour cells are bland, polygonal, forming nests and sheets with abundant granular eosinophilic cytoplasm and small uniform, round to oval nuclei. GCTs do not contain mucin and do not react with cytokeratin or Epithelial Membrane Antigen (EMA) [5]. Most GCTs behave in a benign fashion and surgical excision with clear margins is usually curative. As the lesion has infiltrative margins, hence, incomplete excision may result in local recurrence. An adequate curative resection may be planned on recognition of this lesion on FNAC or frozen section [3]. While the majority (>99%) of GCTs are benign, occasional ones (<1%) are malignant [4,5]. However, benign and malignant GCTs are remarkably similar in histological appearance. Malignant tumours are usually larger in size and the tumour cells are often smaller, elongated and more pleomorphic. In addition, the nuclei are generally hyperchromatic and irregular with an increased nucleus to cytoplasm ratio. Tumour size >4 cm with increased mitotic activity (2 or more/10 high power field) is seen in malignant lesions [3,5]. Also, Malignant GCT is associated with higher recurrence and short survival [6]. In contrast to the other sites, pseudoepitheliomatous hyperplasia of the overlying epidermis cannot be a useful clue in this site.
A few benign lesions which mimic GCT include fat necrosis and mammary duct ectasia whereas malignant lesions include apocrine carcinoma and metastatic carcinomas [4]. Negative staining for histiocyte associated antigens like α1 antitrypsin, α1-antichymotrypsin, and muramidase differentiates GCT from histiocytic tumours. Though, both have an infiltrative pattern, apocrine carcinoma can be differentiated from GCTs based on the presence of overtly malignant nuclear features and adjoining focus of intraductal carcinoma. The IHC in the former will show cytokeratin and epithelial membrane antigen positivity. In addition, uncommon metastatic neoplasms like melanoma and renal cell carcinoma have to be ruled out, using negative staining with pancytokeratin [7].
Conclusion
The GCT is a relatively uncommon lesion of breast and remains a diagnostic challenge for radiologists, surgeons and pathologists as it closely mimics breast carcinoma. Definite diagnosis is, therefore, based on the morphological findings with wide excision of the lesion being the treatment of choice. Specificity of FNA is low as it is a mimic of numerous entities. A preoperative core biopsy or intraoperative frozen section can help surgeons select a conservative surgical procedure.
[1]. Ilvan S, Üstündag N, Calay Z, Bükey Y, Benign granular-cell tumour of the breastCan J Surg 2005 48(2):155-56. [Google Scholar]
[2]. Hammas N, Fatemi HE, Jayi S, Hafid I, Fikri G, El Houari A, Granular cell tumour of the breast: a case reportJ Med Case Rep 2014 8(1):46510.1186/1752-1947-8-46525541096 [Google Scholar] [CrossRef] [PubMed]
[3]. Muzafar S, Siddiqui MS, Kayani N, Lodhi IK, Hasan SH, Granular cell tumour of the breast:an uncommon lesion that mimics carcinomaJ Pak Med Assoc 2000 50(12):426-28. [Google Scholar]
[4]. Adeniran A, Al-Ahmadie HA, Mahoney MC, Robinson-Smith TM, Granular cell tumour of the breast: a series of 17 cases and review of the literatureBreast J 2004 10(6):528-31.10.1111/j.1075-122X.2004.21525.x15569210 [Google Scholar] [CrossRef] [PubMed]
[5]. Pei Z, Wang S, Wang H, Rare breast granular cell tumour with alpha-1 antitrypsin expression: a case report and literature reviewNorth Am J Med Sci 2013 6(2):103-06. [Google Scholar]
[6]. Fanburg-Smith JC, Meis-Kindblom JM, Fante R, Kindblom LG, Malignant granular cell tumour of soft tissue: diagnostic criteria and clinicopathologic correlationAm J Surg Pathol 1998 22(7):779-94.10.1097/00000478-199807000-000019669341 [Google Scholar] [CrossRef] [PubMed]
[7]. Roshong-Denk SL, Montagnese MD, Staren E, Zaher A, Pathologic quiz case: an upper outer quadrant breast mass in a 47-year-old African American woman. Granular cell tumourArch Pathol Lab Med 2003 127(11):1525-26. [Google Scholar]