Introduction
Acute kidney injury is a serious and fatal complication that occurs in 1-2% of patients after cardiac surgery [1,2]. Patients with AKI need dialysis; mortality among these patients is over 60% [1,2]. In less severe AKI, cases that do not need dialysis, short-term mortality increases 19-fold [3]. In mild AKI in which serum creatinine levels increase by 25%, over a long time to 10 years after surgery, mortality is doubled [4].
It is thought that the most important mechanism of kidney injury is intraoperative ischaemia-reperfusion injury. The obtained results from animal studies have indicated that AKI may recur when the therapeutic intervention is performed at the shortest time after surgery or during the beginning time or at the early extension phase of ischaemia-reperfusion injury [5-8].
It has been determined that kidney injuries are partly caused due to lack of specific and real-time sensitive markers that cannot achieve early diagnosis of AKI [9]. Right now, serum creatinine is the AKI diagnostic marker; however, its increase is delayed, which causes postponement of the treatment processes [10,11]. Serum creatinine is not a precise marker for Glomerular Filtration Rate (GFR) in stable condition [12]; therefore, acute changes in serum creatinine at the time of AKI may lack proper precision and may not accurately reflect the severe changes in GFR.
Many studies have been performed in the clinical care and clinical trials to find biomarkers for early diagnosis of AKI with the aim of achieving more time for AKI treatment [13,14]. Although, there have been some advances in this field, creatinine is still the common reference for comparing new biomarkers of kidney injury [15].
Cys C as a novel biomarker is detected in serum earlier than creatinine (about 24-48 hours). Cys C is not affected by tubular secretion and glomerular filtration; so urine Cys C can reflect the glomerular filtration function as well as its serum concentration [16].
Cys C is a much more precise estimator of GFR than creatinine and it has more diagnostic value for chronic kidney disease [12,17]. Some studies have demonstrated the importance of Cys C in early detection of kidney injury after heart surgery [18,19]. Cys C concentration increases within two hours after heart surgery and reaches to a peak at six hours after cardiopulmonary bypass [20].
Another novel marker for AKI is urinary NGAL. Different clinical studies have indicated that increase in serum and urine NGAL is an independent predictor of serum creatinine for AKI diagnosis [21,22]. A meta-analysis had shown the diagnosis and prognosis accuracy of NGAL for AKI identification [9]. Also, it is reported that urinary NGAL significantly reaches to its peak (400 ng/mL) within 2-4 hours after AKI [23]. Similar to Cys C, NGAL can be a better marker than creatinine.
Expression of various MMPs is different in different parts of kidney; for example, MMP-9 is widely expressed in the glomerulus and is considered as a prognostic factor in patients undergoing surgery [24]. The role of MMPs has been demonstrated in the pathophysiology of numerous chronic and acute renal diseases such as renal ischaemic damage. In the development of AKI, increased expression of different MMPs (2, 3, 24, 25, 27, 28, 13, 9 and 14) is reported [24]. Also, an association between cardiac surgery and increased activity of MMPs is proven. Heart surgery induces neutrophil activation, degranulation and some systemic inflammatory responses. The neutrophils contain MMPs and release them during activation [25]. In the present study, we tried to examine three markers and to compare them together and with serum creatinine in patients undergoing cardiac surgery and to find possible biomarkers for early detection of AKI in these patients.
Materials and Methods
This descriptive study was conducted from September to October 2015 among the patients referred to Cardiovascular Surgery Center at Shahid Modarres Hospital in Tehran city, Iran. From all of the patients who underwent open-heart surgery, 29 patients as per the exclusion criteria were selected and included in the study. Exclusion criteria for the study: using nephrotoxic drugs before surgery, history of kidney failure, urinary tract obstruction, consumption of nonsteroidal anti-inflammatory drugs in couple of weeks before surgery or first three days after surgery, history of neoplastic diseases, and history of stroke during six months before surgery.
Written informed consent was obtained from every participant before enrollment. A serum sample at two intervals (6 and 12 hours) after surgery was obtained from all individuals; creatinine was measured in these samples using routine tests. According to increase in serum creatinine, the patients were divided to two groups (AKI and control). AKI group (n=12) had at least 50% increase in serum creatinine; those with less than 50% increase were considered as controls (n=17).
Blood and urine samples were collected from control and AKI patients. After 12 hours of surgery, urine specimens were obtained from all patients, centrifuged for five minutes at 2000 g, and the supernatant was stored in aliquots at -80°C. Serum creatinine was measured using Jaffe’s colorimetric method (Pars Azmun Kit, Iran), according to the kit manufacturer and monitored at least twice a day during the next three days after surgery.
Blood samples were collected from control and AKI groups at 6 and 12 hours after surgery and allowed to clot at room temperature then centrifuged at 1000 g for 10 minutes; serum was separated, aliquoted and kept at -80°C.
The first outcome that was defined as AKI extension was 50% or more increase of serum creatinine from the baseline.
Cys C and NGAL markers were measured using sandwich Enzyme Linked Immunosorbent Assay (ELISA) and enzyme immunoassay (BioVendor, Catalogue. No.: RD191009100 and RD191102200R, respectively) based on manufacturer protocol. Kit sensitivity of Cys C and NGAL was 0.02 ng/mL and 0.25 ng/mL, respectively.
Gelatin Zymography
The activity of MMP-9 was determined using gelatin zymography method. Concentration of serum protein was quantitated by Bicinchoninic Acid (BCA) assay then 10 μg protein from each specimen was subjected to zymography. In summary, electrophoresis (BioRad, USA) was done on 10% polyacrylamide gel (Merck, Germany) containing 0.3% gelatin (Bio-Rad) and under non reducing condition. After electrophoresis, gel was washed two times for one hour in 2.5% Triton X-100 (Sigma) solution to remove SDS (BioBasic), then incubated for 16 hours at 37°C in zymography buffer (Tris-HCl buffer with pH=7.4 containing 10 mM calcium chloride) (Merck, Germany) and stained with 5% Coomassie Brilliant Blue G250. Gel densitometry analysis was performed by the Image J 1.44p application (Wayne Rasband, National Institutes of Health, USA) and MMP-9 activity estimated by the use of gelatin lytic activity [26].
The protocol of this study was approved by ethics committee of Hamadan University of Medical Sciences.
Statistical Analysis
Statistical analysis was done by SPSS v.21. Student t-test was used for comparing the data between two groups; paired t-test was used to compare the data at different times in each group. Correlation analysis was carried out using Pearson test to examine any relationship between the studied factors (NGAL, Cys C, MMP-9). A p-value less than 0.05 was considered significant.
Results
The patient’s demographic characteristics are shown in [Table/Fig-1]. The mean concentration of Cys C at 6 and 12 hours after surgery in control group were 702.58±49.82 ng/mL and 713.37±70.83 ng/mL respectively and the difference was not significant (p=0.865). In AKI group, the mean concentration of Cys C at the same times (6 and 12 hours) was 1626.42±385.73 ng/mL and 1856.13±413.9 ng/mL, respectively and the difference was not significant (p=0.551) [Table/Fig-2a].
Demographic characteristics of the study population.
| Characteristics | AKI (n=12) | Control (n=17) |
|---|
| Age (years) (mean±SD) | 62.45±8.04 | 57.58±15.07 |
| Gender (female/male) | 11/1 | 11/6 |
| BMI (mean±SEM) | 26.02±1.41 | 27.04±1.21 |
| Pumps time (minute) (mean±SEM) | 106.18±10.19 | 114.94±9.41 |
| Cross clamp time | 65.72±8.73 | 79.68±10.06 |
| Ejection fraction | <35% | 3 (25%) | 3 (17.6%) |
| >35% | 9 (75%) | 14 (82.4%) |
Cystatin C levels at different time intervals.
*p-value<0.05, Student t-test
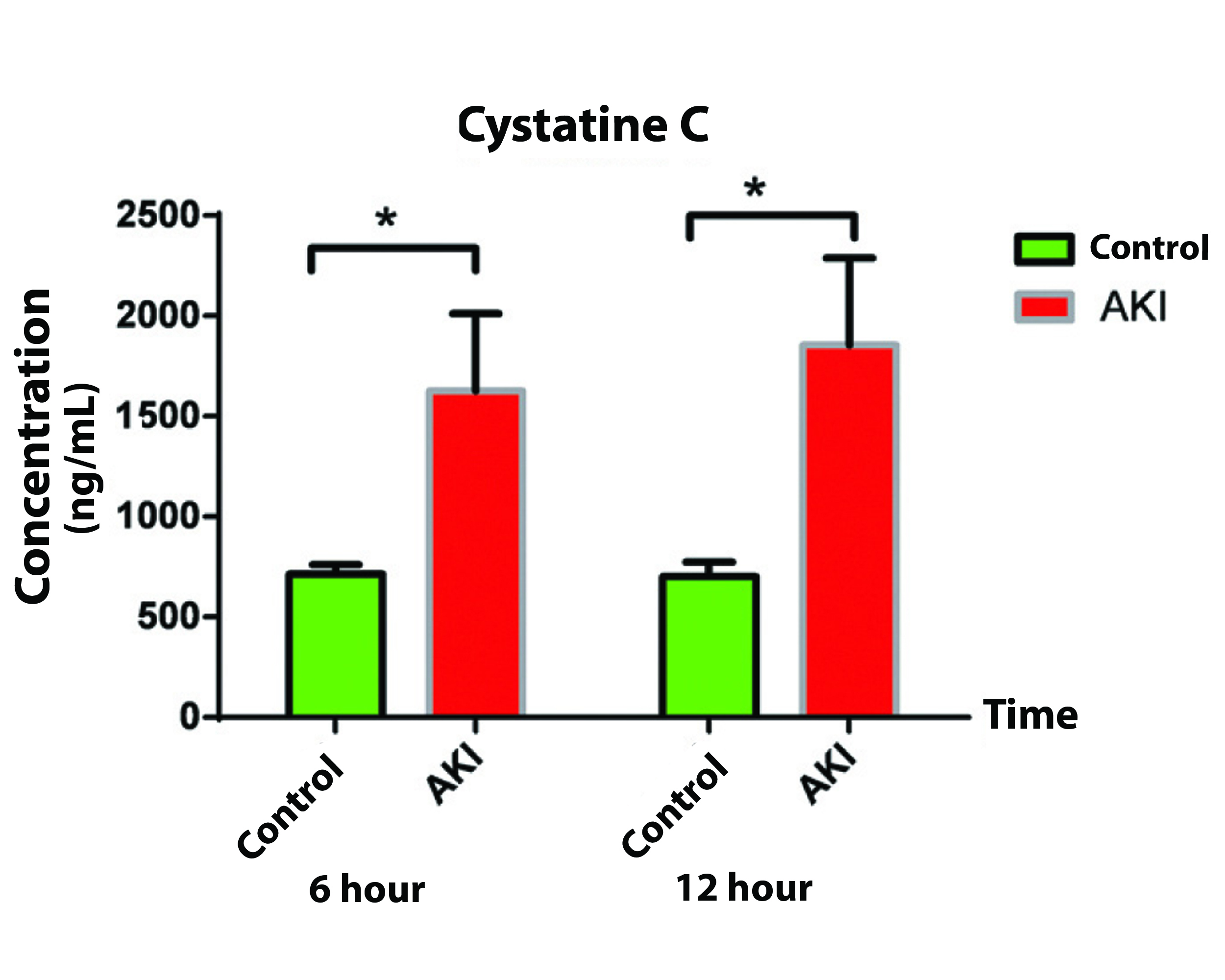
Postoperative Cys C concentration in AKI group showed a remarkable increase compared to that of control group. Cys C concentration comparison between AKI and control group showed significant difference at six hours after surgery (p=0.003) as well as at 12 hours after surgery (p=0.002) [Table/Fig-2b].
Cystatin C comparison between the studied groups at 6 and 12 hour postoperatively.
| Time (hours) | Group | Mean | SEM | p-value |
|---|
| Cystatin C (ng/mL) | 6 | Control | 702.58 | 49.82 | 0.003 |
| AKI | 1626.42 | 385.73 |
| 12 | Control | 713.37 | 70.83 | 0.002 |
| AKI | 1856.13 | 431.90 |
SEM: Std. error mean; p-value was calculated using Student t-test; p-value <0.05 was considered significant.
The mean activity of MMP-9 in control group at 6 and 12 hour after surgery were 1.91 AU and 1.92 AU, respectively and did not show any significant difference (p=0.790). In contrast to control group, in AKI group there was a significant difference in MMP-9 activity between 6 and 12 hours after surgery (2.05 AU and 1.57 AU respectively, p=0.002) [Table/Fig-3a].
MMP levels at different time intervals.
p-value was calculated using paired t-test; **p-value <0.01 was considered significant
*p-value <0.05, Student t-test
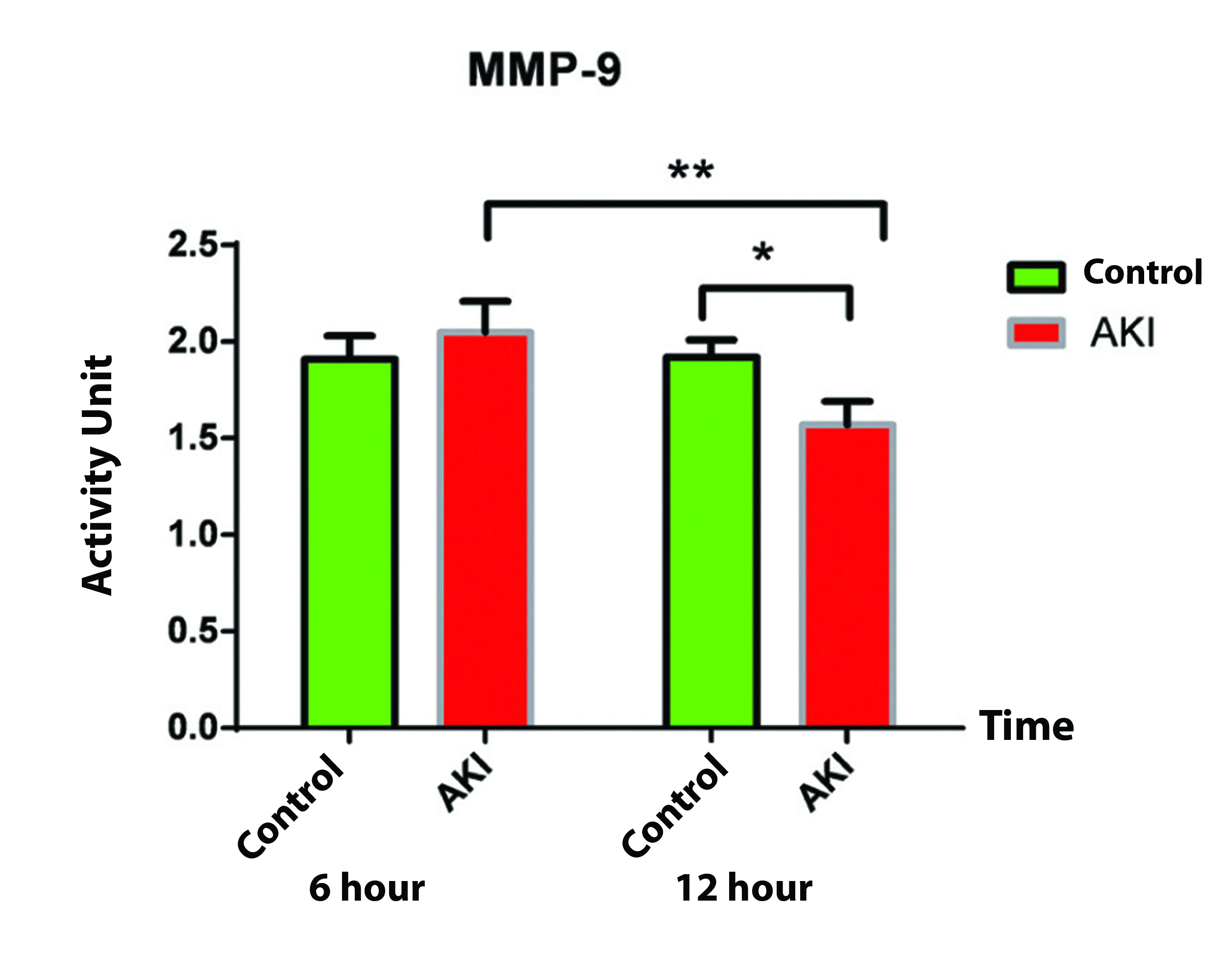
The MMP-9 activity comparison at six hours postoperation did not show any significant difference between AKI and control groups (p=0.517); however, a reduced activity in AKI group was observed at 12 hours postoperation that was significantly different compared to that of control group (p=0.03) [Table/Fig-3b]. Also, the observed reduction in MMP-9 activity was significant at 12 hours compared to six hours postoperation in AKI group.
MMP-9 activity comparison between the studied groups at 6 and 12 hours postoperatively.
| Paired samples statistics |
|---|
| Groups | Time (hours) | Mean | N | p-value |
|---|
| MMP-9 | Control | 6 | 1.91 | 17 | 0.517 |
|---|
| AKI | 2.05 | 12 |
| Control | 12 | 1.92 | 17 | 0.030 |
| AKI | 1.57 | 12 |
p-value <0.05, Student t-test
The comparison of mean concentration of urine NGAL did not show significant difference after surgery in AKI comparing to control group (p=0.879) [Table/Fig-4].
Urinary NGAL comparison between two groups 12 hours postoperatively. No significant difference was observed between two groups.
| Group | Mean | SEM | p-value |
|---|
| NGAL (ng/mL) | Control | 19.41 | 4.33 | 0.879 |
| AKI | 26.34 | 11.20 |
SEM: Std. error mean; Student t-test; p<0.05 was considered significant
There was a positive correlation between urine NGAL and creatinine concentration in both pre and postoperative days (p=0.008, r=-0.48 and p=0.046 and r=+0.37, respectively), but there was no significant correlation between MMP-9 activity and Cys C concentration with serum creatinine concentration changes (p=0.979, r=+0.005; p=0.907, r=-0.023; p=0.367, r=-0.174; p=0.549, r=-0.116, respectively). None of the studied markers showed a significant relation with age, gender, BMI, pumps time, cross clamp time, and ejection fraction variables (p>0.05).
Cys C concentration in patients after surgery had significant and direct correlation with the length of stay in ICU (p=0.002, r=+0.54). There was a negative significant correlation between MMP-9 activity and serum creatinine at 12 hours postoperation (p=0.04, r=-0.50) in control group.
Discussion
Serum creatinine is not a real-time reflector of GFR because it must accumulate in serum as a result of GFR reduction that can 7be detectable [27]. For this reason, the minimum required time for its increase in serum is 24-48 hours and consequently this marker has some limitations for early diagnosis of AKI. Researchers have identified some new markers in the recent years that have an appropriate sensitivity and prediction power for AKI incidence after heart surgery [28,29].
In the present study, the changes in some biomarkers including MMP-9, Cys C and NGAL were studied in patients who underwent cardiac surgery. The results showed that the MMP-9 activity in AKI group after open-heart surgery did not increase; however a remarkable reduction was observed after 12 hours (2.05 to 1.57 AU) (p-value=0.002). This was different from what was observed in control group. In the latter group, no significant changes were observed between two interval times (6 and 12 hours) (p-value=0.790). The present study could not identify the primary cause of MMP-9 activity reduction in AKI group, but based on the results observed in two independent samples it could conceivably be hypothesised that: 1) it is likely that an unknown pathway exists at the time of high creatinine level that is implicated in the controlling of gene expression and/or activity of the MMP-9; and 2) the peak of MMP-9 concentration after heart surgery is in the early hours after surgery (2-6 hours) and after this time the concentration of MMP-9 gradually reduces. The latter hypothesis was supported by other studies that indicated MMP-9 reduction at 12 hour postoperation [25,30].
The scientists developed a unilateral ischaemia model on rats in order to consider the effect of acute ischaemia on gelatinase activity and expression in kidney; they concluded that ischaemia reperfusion injury increases the serum creatinine level and it was considered as a marker for diagnosis of kidney injury [31,32]. Ischaemia reperfusion injury has been known as a strong potential stimulus for induction of mRNA and protein expression of MMP-9 in both monomer and dimer forms [31,32]. These researchers by doing zymography in another study showed that ischaemia reperfusion injury strongly increases the pro-MMP-2 proteolytic activity to 1.8 folds pro-MMP-9 to 3 folds, and MMP-9 to 4 folds [31,32]. These findings indicated that ischaemia-reperfusion injury in kidney can lead to increase basement membrane destruction and blood vessels permeability [31,32].
Cys C has been considered as an early biomarker for AKI diagnosis and it has been shown that it increases before serum creatinine [14,33]; besides, it has been mentioned that Cys C concentration is less affected by the volume status than serum creatinine [34]. There are some reports that show serum Cys C elevation occurs before slight reduction of GFR (one or two days before beginning of symptoms), and even before increase in serum creatinine and kidney function reduction [35,36]. The primary increase of urinary Cys C level is a good predictor for AKI after heart surgery [37].
Some studies indicated Cys C concentration can immediately predict moderate and very severe AKI development after heart surgery [38]. Zappitelli M et al., showed that AKI, as defined by severe increase of Cys C, has a stronger correlation with the two urinary markers of kidney injury (IL-18 and KIM-1) than serum creatinine [39].
The finding of the present study indicated that in AKI group compared with control group, serum Cys C concentration was higher and this result showed that injury in kidney (at any level) leads to increase in serum Cys C and creatinine. The comparison of serum Cys C concentration at 6 hours postoperation between control and AKI group indicated a significant increase in AKI group (p-value=0.003). This result indicated that Cys C can be a good marker for AKI diagnosis in patients after open-heart surgery. On the other hand, since the comparison of Cys C concentration did not show a significant difference at 6 and 12 hours postoperation among AKI group, it can be concluded that this marker may increase in early hours after surgery and the rate of this increase may be slower after six hours, because it did not increase significantly at 12 hours postoperation. Therefore, it can be concluded that Cys C is an appropriate early marker to consider for kidney function after open-heart surgery; this marker can be measured in the initial hours (before 12 hours) postoperation for rapid and early detection of GFR changes. The obtained results were in line with some previous studies that reported serum Cys C has enough power and sensitivity in early diagnosis of acute kidney failure after heart surgery [40-42]; even Cys C serum concentration after surgery is used as an appropriate marker to classify AKI patients (1 and 2 stages) for treatment [38].
A number of studies demonstrate conflicting results about the early detection of AKI after cardiac surgery by Cys C. Zappitelli M et al., reported that identifying AKI by Cys C is much later than by creatinine (the average diagnosis time 48 and 24 hours, respectively) [39]; however, most of these patients were in Stage I AKI which was verified by creatinine assay. The reason of increased creatinine in these patients may be due to increased production, secretion and metabolism of creatinine, and not exactly due to renal tubular injury. Their theory was supported by the correlation between Cys C and some biomarkers that had been studied; therefore, they concluded that even though the mean Cys C is identifiable after creatinine, it does not essentially mean that Cys C is a delayed biomarker [39].
In the present study, NGAL in urine after open-heart surgery was considered as a contributory factor in early diagnosis of kidney injuries. Although, this marker had a relative increase in AKI group compared to control group after surgery, its changes were not statistically significant (p-value=0.879); however, this result showed that using this marker could not be a good alternative for creatinine in kidney injury diagnosis, this result was in contrast with some published reports [9,23].
Our results indicated that urinary NGAL had a very little ability in AKI diagnosis after heart surgery and it was in line with other studies [37,43]. It was indicated in a review that the common markers in the best situation have a low diagnostic power for AKI detection in short time after heart surgery in adults [29]. In another review study, Hjortrup PB et al., realised that different study results are very diverse in early identification of AKI by NGAL; these results are different from that which indicated NGAL is worthless to have a good diagnostic value in the field of AKI [27]. Watanabe M et al., observed a positive correlation between serum creatinine and urinary NGAL in AKI patients; the highest concentration of serum creatinine correlated to higher values of urinary NGAL [44]. These results were in line with this study finding, that showed a positive correlation between urinary NGAL and serum creatinine before and after surgery.
Limitation
One of the important limitation of this study was the small number of patients in each group, also the small study duration; therefore, it can be considered as a preliminary report.
Conclusion
It can be concluded that Cys C and MMP-9 can be the reliable markers for early detection of AKI comparing to serum creatinine in patients after cardiac surgery. According to the obtained data, NGAL cannot be recommended as a sensitive marker in these patients.
SEM: Std. error mean; p-value was calculated using Student t-test; p-value <0.05 was considered significant.
p-value <0.05, Student t-test
SEM: Std. error mean; Student t-test; p<0.05 was considered significant
[1]. Chertow GM, Lazarus JM, Christiansen EF, CHammermeisterook KE, Grover F, Preoperative renal risk stratificationCirculation 1997 95(4):878-84.10.1161/01.CIR.95.4.8789054745 [Google Scholar] [CrossRef] [PubMed]
[2]. Thakar CV, Arrigain S, Worley S, Yared J-P, Paganini EP, A clinical score to predict acute renal failure after cardiac surgeryJ Am Soc Nephrol 2005 16(1):162-68.10.1681/ASN.200404033115563569 [Google Scholar] [CrossRef] [PubMed]
[3]. Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT, Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilizationAnn Intern Med 1998 128(3):194-203.10.7326/0003-4819-128-3-199802010-000059454527 [Google Scholar] [CrossRef] [PubMed]
[4]. Loef BG, Epema AH, Smilde TD, Henning RH, Ebels T, Navis G, Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survivalJ Am Soc Nephrol 2005 16(1):195-200.10.1681/ASN.200310087515563558 [Google Scholar] [CrossRef] [PubMed]
[5]. Devarajan P, Update on mechanisms of ischemic acute kidney injuryJ Am Soc Nephrol 2006 17(6):1503-20.10.1681/ASN.200601001716707563 [Google Scholar] [CrossRef] [PubMed]
[6]. Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalinJ Am Soc Nephrol 2004 15(12):3073-82.10.1097/01.ASN.0000145013.44578.4515579510 [Google Scholar] [CrossRef] [PubMed]
[7]. Bellomo R, Kellum J, Ronco C, Defining acute renal failure: physiological principlesIntensive Care Med 2004 30(1):33-37.10.1007/s00134-003-2078-314618231 [Google Scholar] [CrossRef] [PubMed]
[8]. Bonventre JV, Weinberg JM, Recent advances in the pathophysiology of ischemic acute renal failureJ Am Soc Nephrol 2003 14(8):2199-210.10.1097/01.ASN.0000079785.13922.F612874476 [Google Scholar] [CrossRef] [PubMed]
[9]. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A, Group NM, Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysisAm J Kidney Dis 2009 54(6):1012-24.10.1053/j.ajkd.2009.07.02019850388 [Google Scholar] [CrossRef] [PubMed]
[10]. Bellomo R, Ronco C, Kellum JA, Palevsky RL, Acute dialysis quality ]initiative workgroup. Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) groupCrit Care 2004 8(4):R204-12. [Google Scholar]
[11]. Berl T, American society of nephrology renal research reportJ Am Soc Nephrol 2005 [Google Scholar]
[12]. Schwartz GJ, Work DF, Measurement and estimation of GFR in children and adolescentsClin J Am Soc Nephrol 2009 4(11):1832-43.10.2215/CJN.0164030919820136 [Google Scholar] [CrossRef] [PubMed]
[13]. Koyner JL, Parikh CR, Clinical utility of biomarkers of AKI in cardiac surgery and critical illnessClin J Am Soc Nephrol 2013 8(6):1034-42.10.2215/CJN.0515051223471130 [Google Scholar] [CrossRef] [PubMed]
[14]. Kiessling AH, Dietz J, Reyher C, Stock UA, Beiras-Fernandez A, Moritz A, Early postoperative serum cystatin C predicts severe acute kidney injury following cardiac surgery: a post-hoc analysis of a randomized controlled trialJ Card Surgery 2014 988(1):1010.1186/1749-8090-9-1024397879 [Google Scholar] [CrossRef] [PubMed]
[15]. James M, Bouchard J, Ho J, Klarenbach S, LaFrance JP, Rigatto C, Canadian society of nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injuryAm J Kidney Dis 2013 61(5):673-85.10.1053/j.ajkd.2013.02.35023518195 [Google Scholar] [CrossRef] [PubMed]
[16]. Bekier Z, elawska A, Kokot M, Biolik G, Ziaja D, Ziaja K, Janowska M, Cystatin C as potential marker of acute kidney injury in patients after abdominal aortic aneurysms surgery-preliminary studyAnn Acad Med Siles 2014 68(1):9-15. [Google Scholar]
[17]. Filler G, Lepage N, Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula?Pediatr Nephrol 2003 18(10):981-85.10.1007/s00467-003-1271-512920638 [Google Scholar] [CrossRef] [PubMed]
[18]. Liang X, Shi W, Liu S, Yan L, Xuan H, Xiong W, Prospective study of cystatin C for diagnosis of acute kidney injury after cardiac surgeryJ South Med Uni 2008 28(12):2154-56. [Google Scholar]
[19]. Moore E, Bellomo R, Nichol A, Biomarkers of acute kidney injury in anesthesia, intensive care and major surgery: from the bench to clinical research to clinical practiceMinerva Anestesiologica 2010 76(6):425-40. [Google Scholar]
[20]. Seitz S, Rauh M, Gloeckler M, Cesnjevar R, Dittrich S, Koch A, Cystatin C and meutrophil gelatinase-associated lipocalin: biomarkers for acute kidney injury after congenital heart surgerySwiss Med Wkly 2013 143:w1374410.4414/smw.2013.1374423348800 [Google Scholar] [CrossRef] [PubMed]
[21]. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A, Meta-analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysisAm J Kidney Dis 2009 54(6):1012-24.10.1053/j.ajkd.2009.07.02019850388 [Google Scholar] [CrossRef] [PubMed]
[22]. Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgeryThe Lancet 2005 365(9466):1231-38.10.1016/S0140-6736(05)74811-X [Google Scholar] [CrossRef]
[23]. Xin C, Yulong X, Yu C, Changchun C, Feng Z, Xinwei M, Urine neutrophil gelatinase-associated lipocalin and interleukin-18 predict acute kidney injury after cardiac surgeryRenal Failure 2008 30(9):904-13.10.1080/0886022080235908918925531 [Google Scholar] [CrossRef] [PubMed]
[24]. Catania JM, Chen G, Parrish AR, Role of matrix metalloproteinases in renal pathophysiologiesAmerican Journal of Physiology-Renal Physiology 2007 292(3):F905-F11.10.1152/ajprenal.00421.200617190907 [Google Scholar] [CrossRef] [PubMed]
[25]. Lin T-C, Li C-Y, Tsai C-S, Ku C-H, Wu C-T, Wong C-S, Neutrophil-mediated secretion and activation of matrix metalloproteinase-9 during cardiac surgery with cardiopulmonary bypassAnesth Analg 2005 100(6):1554-60.10.1213/01.ANE.0000154307.92060.8415920174 [Google Scholar] [CrossRef] [PubMed]
[26]. Hawkes SP, Li H, Taniguchi GT, Zymography and reverse zymography for detecting MMPs and TIMPs. Matrix metalloproteinase protocolsMethods Mol Biol 2010 622:257-69.10.1007/978-1-60327-299-5_1620135288 [Google Scholar] [CrossRef] [PubMed]
[27]. Hjortrup PB, Haase N, Wetterslev M, Perner A, Clinical review: predictive value of [27]neutrophil gelatinase-associated lipocalin for acute kidney injury in intensive care patientsCrit Care 2013 17(2):21110.1186/cc1185523680259 [Google Scholar] [CrossRef] [PubMed]
[28]. Gabbard W, Milbrandt EB, KKellum JA, NGAL: an emerging tool for predicting severity of AKI is easily detected by a clinical assayCrit Care 2010 14(4):1-2.10.1186/cc907120804576 [Google Scholar] [CrossRef] [PubMed]
[29]. Ho J, Tangri N, Komenda P, Kaushal A, Sood M, Brar R, Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysisAm J Kid Dis 2015 66(6):993-1005.10.1053/j.ajkd.2015.06.01826253993 [Google Scholar] [CrossRef] [PubMed]
[30]. Sokal A, Zembala M, Radomski A, Kocher A, Pacholewicz J, Los J, A differential release of matrix metalloproteinases 9 and 2 during coronary artery bypass grafting and off-pump coronary artery bypass surgeryJ Thorac Cardiovasc Surg 2009 137(5):1218-24.10.1016/j.jtcvs.2008.11.00419379995 [Google Scholar] [CrossRef] [PubMed]
[31]. Caron A, Desrosiers RR, Beliveau R, Ischemia injury alters endothelial cell properties of kidney cortex: stimulation of MMP-9Exp Cell Res 2005 310(1):105-16.10.1016/j.yexcr.2005.07.00416112109 [Google Scholar] [CrossRef] [PubMed]
[32]. Caron A, Desrosiers RR, Langlois S, Beliveau R, Ischemia-reperfusion injury stimulates gelatinase expression and activity in kidney glomeruliCan J Physiol Pharmacol 2005 83(3):287-300.10.1139/y05-01115870843 [Google Scholar] [CrossRef] [PubMed]
[33]. Ataei N, Bazargani B, Ameli S, Madani A, Javadilarijani F, Moghtaderi M, Early detection of acute kidney injury by serum cystatin C in critically ill childrenPediatr Nephrol 2014 29(1):133-38.10.1007/s00467-013-2586-523989306 [Google Scholar] [CrossRef] [PubMed]
[34]. Soto K, Coelho S, Rodrigues B, Martins H, Frade F, Lopes S, Cystatin C as a marker of acute kidney injury in the emergency departmentClin J Am Soc Nephrol 2010 5(10):1745-54.10.2215/CJN.0069011020576828 [Google Scholar] [CrossRef] [PubMed]
[35]. Herget-Rosenthal S, Marggraf G, Hüsing J, Gğring F, Pietruck F, Janssen O, Early detection of acute renal failure by serum cystatin CKidney Int 2004 66(3):1115-22.10.1111/j.1523-1755.2004.00861.x15327406 [Google Scholar] [CrossRef] [PubMed]
[36]. Pirgakis KM, Makris K, Dalainas I, Lazaris AM, Maltezos CK, Liapis CD, Urinary cystatin C as an early biomarker of acute kidney injury after open and endovascular abdominal aortic aneurysm repairAnn Vasc Surg 2014 28(7):1649-58.10.1016/j.avsg.2014.04.00624858592 [Google Scholar] [CrossRef] [PubMed]
[37]. Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgeryKidney Int 2008 74(8):1059-69.10.1038/ki.2008.34118650797 [Google Scholar] [CrossRef] [PubMed]
[38]. Zappitelli M, Krawczeski CD, Devarajan P, Wang Z, Sint K, Thiessen-Philbrook H, Early postoperative serum cystatin C predicts severe acute kidney injury following pediatric cardiac surgeryKidney Int 2011 80(6):655-62.10.1038/ki.2011.12321525851 [Google Scholar] [CrossRef] [PubMed]
[39]. Zappitelli M, Greenberg JH, Coca SG, Krawczeski CD, Li S, Thiessen-Philbrook HR, Association of definition of acute kidney injury by cystatin C rise with biomarkers and clinical outcomes in children undergoing cardiac surgeryJAMA pediatrics 2015 169(6):583-91.10.1001/jamapediatrics.2015.5425844892 [Google Scholar] [CrossRef] [PubMed]
[40]. Krawczeski CD, Vandevoorde RG, Kathman T, Bennett MR, Woo JG, Wang Y, Serum cystatin C is an early predictive biomarker of acute kidney injury after pediatric cardiopulmonary bypassClin J Am Soc Nephrol 2010 5(9):1552-57.10.2215/CJN.0204031020538834 [Google Scholar] [CrossRef] [PubMed]
[41]. Wald R, Liangos O, Perianayagam MC, Kolyada A, Herget-Rosenthal S, Mazer CD, Plasma cystatin C and acute kidney injury after cardiopulmonary bypassClin J Am Soc Nephrol 2010 5(8):1373-79.10.2215/CJN.0635090920522534 [Google Scholar] [CrossRef] [PubMed]
[42]. Zhang Z, Lu B, Sheng X, Jin N, Cystatin C, C in prediction of acute kidney injury: a systemic review and meta-analysisAm J Kidney Dis 2011 58(3):356-65.10.1053/j.ajkd.2011.02.38921601330 [Google Scholar] [CrossRef] [PubMed]
[43]. Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT, Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgeryAm J Kidney Dis 2008 52(3):425-33.10.1053/j.ajkd.2008.05.01818649981 [Google Scholar] [CrossRef] [PubMed]
[44]. Watanabe M, Fulan e Silva G, Dezoti da Fonseca C, Vattimo MdFF, Urinary NGAL in patients with and without acute kidney injury in a cardiology intensive care unitRev Bras Ter Intensiva 2014 26(4):347-54.10.5935/0103-507X.2014005325607262 [Google Scholar] [CrossRef] [PubMed]