Introduction
Alzheimer’s disease is one of the most common neurodegenerative disease and accounts for more than 80% of dementia cases worldwide in elderly people; it leads to the progressive loss of memory and cognitive decline and ability to learn [1]. The cause of dementia can vary, depending on the types of brain changes like frontal temporal dementia, lewy body dementia and vascular dementia. Studies suggest that metabolically significant vitamin B12 (cobalamin) and folate (folic acid) deficiency in the elderly are much more common than is widely believed [2]. These deficiencies in the elderly are important because these vitamins are crucial for proper brain function and play an important role in mental and emotional health. Metabolism of folate and vitamin B12 is interconnected, deficiency of either, commonly produces megaloblastic anaemia and sometimes severe neurological consequences [3] though folate is involved in one carbon metabolism, it also acts as a cofactor in many different biochemical reaction [4]. Humans cannot synthesise folate and therefore we must obtain it in diet. Major sources of folate are green vegetables, citrus fruits, liver and whole grains. The main dietary folates are 5-methyltetrahydrofolate and formyltetrahydrofolate, which are readily transported across the intestinal epithelium. Vitamin B12-dependent methionine synthase plays an important role in facilitating the conversion of extracellular 5-methyltetrahydrofolate to monoglutamyltetrahydro folate, a form of folate that can be readily used in nucleotide biosynthesis. Indeed, one consequence of folate deficiency in humans is an increased risk of cancers.
Evidence suggests that low vitamin D levels also play a role in the pathogenesis of a wide range of non skeletal age-associated diseases such as various cancers [5], type 2 diabetes [6], cardiovascular disease [7], hypertension [8] and stroke [9]. Low serum 25-hydroxyvitamin D (25(OH)D) levels, a stable marker of vitamin D status, are also associated with increased odds of prevalent cognitive dysfunction and dementia in a number of studies, raising the possibility that vitamin D plays a role in the aetiology of cognitive dysfunction, Alzheimer’s disease and all-cause dementia [10,11]. The role of plasma homocysteine, folate and vitamin B12 levels with the cognitive status of the patient suffering from SDAT have not been evaluated so far in Varanasi. The area of the country where this study has been conducted is comparatively an economically deprived section. Hence, this study will help in throwing light on the preventive aspect of dementia in terms of nutritional supplementation like vitamin B12, folate. Hence, in present study an attempt was made to assess correlation between plasma homocysteine, folate and vitamin B12 levels with cognitive status of patient suffering from SDAT. It is called senile dementia because it is age related and higher incidence in elderly age group more than 60 years.
Materials and Methods
The present study was carried out in the Department of Biochemistry in association with the Department of Psychiatry at the Institute of Medical Sciences, BHU, Varanasi, Uttar Pradesh, India. A total of
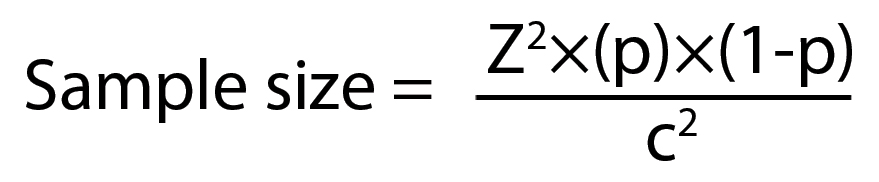
110 patients suffering from SDAT were taken. The sample size was calculated using formula:
Where:
Z = Z-value (e.g., 1.96 for 95% confidence level)
p = percentage picking a choice, expressed as decimal
(0.5 used for sample size needed)
c = confidence interval, expressed as decimal
(e.g., 0.04=±4)
Study included elderly patient aged 60-75 years with confirmed clinical diagnosis of SDAT with the help of CDR (Clinical Dementia Reading) scale [12] between December 2014 to May 2016. CDR is a semi-structured interview in which questionnaire regarding the personal information of the patients, occupation, home, hobbies, memory have been asked. It was conducted in the native language, Hindi. The duration of interview was 40-45 minutes for each patient.
The power of the study was p<0.001 and the CI was 95. The interview was taken by the Chief Investigator (first author) along with the Psychiatrist (fifth author), in the OPD of the Department of Psychiatry where there are separate cubicles for interview and examination of patient. Ethical clearance (EC Registration No. ECR/526/Inst/UP/2014 Dt.31.1.14) was taken from Institute Ethics Committee before beginning the study and informed consent was taken before collection of blood samples from cases and controls.
Inclusion criteria: All the study subjects were diagnosed by the consultant psychiatric according to DSM IV and NINCDS-ADRDA criteria [13]. Patients having dementia as rated on Clinical Dementia Rating (CDR) scale.
Exclusion criteria: Patient suffering from any significant comorbid physical illness, clinically diagnosed DSM V psychiatric illness, any nutrition deficient status or patient not accompanied by any care giver.
Blood samples were collected from patient upon admission taking all aseptic precautions, about 5 mL of blood was drawn by venipuncture from a peripheral vein, with a disposable syringe and thereafter transferred to a clean dry glass tube where it was allowed to stand for 30 minutes for retraction of clot. This was centrifuged at 3000 rpm for 10 minutes to separate the serum. The serum sample was stored at -20°C in the refrigerator for analysis. Care was taken to avoid haemolysis of sample.
The study comprised of two groups: Group 1 consisted of 110 cases which were diagnosed with SDAT age between 60-75 year. This group was given oral supplementation of vitamin B12, folate along with the treatment prescribed by the treating psychiatrist (MS) i.e., TAU
Group 2 consisted 55 cases of with SDAT age between 60-75 year and were given no oral supplementation, but were continued on TAU. These patients were taken as controls.
This helped in comparing the groups with supplementation and without supplementation the variable being the cognitive status i.e., (memory, language and skills).
Estimation of serum vitamin B12 and folate: Quantitative measurement of serum vitamin B12, folate, and vitamin D was done 2by competitive ELISA. ELISA kit for vitamin B12 (Cat no.-E-EL-0010, Lot no.-AK0016JUL04021), kit for FA/vitamin B9 (Cat no.-E-EL-0009, Lot no. - AK0016JUL04020), kit for vitamin D (Cat no. E-EL-0012, Lot no. AK0016JUL04022) uses Competitive-ELISA as the method. The microtiter plate provided in kit has been pre-coated with vitamin B12. During the reaction, vitamin B12 in the sample or standard, competes with a fixed amount of vitamin B12 on the solid phase supporter so as to label antibodies (biotinylation) for use, regarding vitamin B12. Excess conjugate and unbound sample or standard are washed from the plate, and Avidin conjugated to Horseradish Peroxidase (HRP) was added to each microplate well and incubated. Then, a TMB substrate solution was added to each well. The enzyme-substrate reaction was terminated by the addition of a sulphuric acid solution and the colour change was measured spectrophotometrically at a wavelength of 450 nm±2 nm. The concentration of vitamin B12 in the samples was then determined by comparing the OD of the samples to the standard curve. The same procedure was repeated for folate.
Estimation of serum homocysteine: Kit (Cat No. E3292Hu, Lot no.-20160623) uses ELISA based on the Biotin double antibody sandwich technology to assay the Human Homocysteine (Hcy). Hcy was added to the wells, which was pre-coated with Hcy monoclonal antibody and then incubated. After that, anti Hcy antibodies labelled with biotin was added to unite with streptavidin-HRP, which forms immune complex. Unbound enzymes were removed after incubation and washing. Substrate A and B was then added. The solution turned blue and changed into yellow with the effect of acid. The shades of solution and the concentration of Human Hcy were positively correlated.
Estimation of vitamin D: Kit for Vitamin D (Cat no. E-EL-0012, Lot no. AK0016JUL04022) uses competitive-ELISA as the method. The microtiter plate provided in kit has been pre-coated with vitamin D. During the reaction, vitamin D in the sample or standard competes with a fixed amount of vitamin D on the solid phase supporter for sites on the Biotinylated or labelled antibody detection sites which are specific to vitamin D. Excess conjugate and unbound sample or standard are washed from the plate, and Avidin conjugated to HRP was added to each microplate well and incubated. Then, a TMB substrate solution was added to each well. The enzyme-substrate reaction was terminated by the addition of a sulphuric acid solution and the colour change was measured spectrophotometrically at a wavelength of 450 nm±2 nm. The concentration of vitamin D in the samples was then determined by comparing the OD of the samples to the standard curve.
Statistical Analysis
Statistical analysis was done between patients of SDAT and age matched controls using SPSS-20.0 and R studio software. The data were expressed as mean±SD, p<0.005 was considered significant and mean±SD, p<0.001 was highly significant.
Results
The present study was carried out to evaluate the role of homocysteine, folate, vitamin B12 and vitamin D and its effect on cognitive status of patient suffering from SDAT. The levels before supplementation were as follows: vitamin B12- 1.02 ng/mL, Folate- 16.20 ng/mL, vitamin D- 18.10 ng/mL, Homocysteine-10.06 nm/mL in cases. Out of the 110 cases, CDR scales of 97 cases did not change after vitamin B12 and folate supplementation for one month. CDR scale of 10 patients deteriorated by a fraction of 0.5 to 1 whereas CDR scales for 3 cases improved by 0.5 point.
The observation showed that vitamin B12 and folate supplementations do not improve CDR scale significantly (p>0.05). Estimation of homocysteine was expressed as nm/mL. The mean serum homocysteine in cases was 11.65±8 nm/mL and in controls was 6.27±6.77 nm/mL. The homocysteine level when compared between cases and controls was found to be highly significant (p<0.001).
Serum folate, vitamin D and vitamin B12 were estimated by competitive ELISA method as described under materials and methods [Table/Fig-1]. The mean level was lower in cases as compared to controls. The serum folate (pre 17.59±22.74 ng/mL/post 26.65±27.26 ng/mL), vitamin B12 (pre 1.27±1.61 ng/mL/post 1.44±0.75 ng/mL) and vitamin D (pre 21.01±34.68 ng/mL/post 42.74±70.49 ng/mL) level in cases was found not significant when compared to controls. Vitamin B12, B9 and vitamin D levels were significantly decreased in cases compared to controls. However, the trend of decrease can be examined by using a bigger sample size.
Vitamin B12, folate, vitamin D and homocysteine levels in controls and cases with alzheimer’s disease (mean±SD).
| Parameters | Mean±SD Case (110) | Mean±SD Control (55) |
|---|
| Vitamin B12 (ng/mL) p<0.05 | 1.27±1.61 | 1.44±0.75 |
| Folate (ng/mL) p<0.05 | 17.59±22.74 | 26.65±27.26 |
| Vitamin D (ng/mL) p<0.05 | 21.01±34.68 | 42.74±70.49 |
| Homocysteine (nm/mL) p<0.001 | 11.65±8 | 6.27±6.77 |
Vitamin B12, Folate and Vitamin D levels were not significant as compared to controls, whereas Homocysteine level was highly significant as compared to controls.
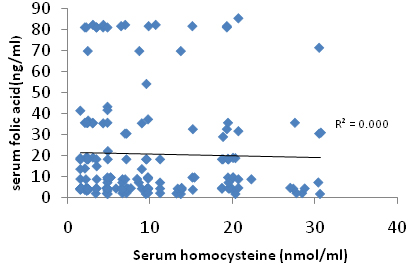
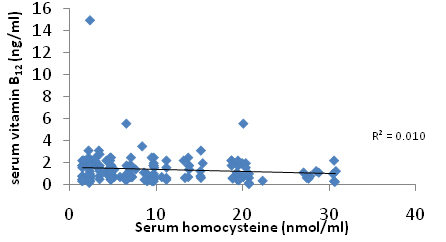
A correlation plot [Table/Fig-2] between serum folate and homocysteine [Table/Fig-3] serum homocysteine and vitamin B12 shows that there was no correlation between these parameters and it was observed that these parameters are not related with each other and was statistically insignificant. No correlation was done in between vitamin D and homocysteine.
Discussion
In the present study, 110 Alzheimer’s disease patients and 55 age matched controls were taken. It was observed that serum levels of homocysteine are significantly higher in patients in comparison to age matched controls. Vitamin D was significantly lower among the patients in comparison to controls. Studies suggested that metabolically significant vitamin B12 and folate deficiency in elderly is commonly present and the extent is more than what is reported in the literature [2,14]. These vitamins are required for proper brain function. These are interdependent and essential component of the one carbon metabolism. Homocysteine is produced from methionine by demethylation. Homocysteine levels are normally maintained low. The mechanism in which homocysteine is remethylated to form methionine by a reaction that requires folate and vitamin B12 [15]. Therefore, deficiency of vitamin B9 and vitamin B12 increases homocysteine level.
In present study, the serum homocysteine levels in patients with Alzheimer’s disease were significantly higher than controls (p<0.001). As vitamin B12 and folic acid deficiency also increase the homocysteine levels in our body, these vitamins help in conversion of homocysteine to cysteine. There is familial form of vitamin B12 deficiency induced Alzheimer’s disease which is not associated with other sign and symptoms of vitamin B12 deficiency which include macrocytosis and anaemia [11]. Vitamin B12 and folate levels in patients and controls were studied but both the levels were statistically insignificant (p>0.005). Vitamin D deficiency is a risk factor for cardiovascular diseases so it may be associated with increased risk with dementia and Alzheimer’s disease. Its deficiency is associated with increased plaque and tangles formation in the brain, so increased risk of Alzheimer’s disease. In present study, vitamin D levels were significantly decreased in cases in comparisons to the controls (p<0.001 i.e., highly significant). A correlation plot [Table/Fig-2,3] was then drawn to observe the correlation between these parameters and it was observed that these parameters are not related with each other and that was statistically insignificant. The above study is important in its attempt to correlate the vitamins B12, folate and vitamin D with the cognitive status. The homocysteine levels have also been correlated with the cognitive status. We have used well standardised procedures to assess the cognitive status and also the biochemical levels.
Plot showing correlation between serum homocysteine and serum folic acid.
Plot showing correlation between serum homocysteine and vitamin B12.
Limitation
Present study had certain limitations like the sample size was small because a bigger sample size can help in robust statistical analysis. The second limitation is that we could not entirely control and measure the dietary intake of the study subjects. The limitations of the present study were the small duration of supplementation (1 month); hence we could not see any change in the cognitive status of the study subjects. Another limitation of the study was that it was a clinic based study and the status of community dwelling subjects still needs to be investigated. Finally, the study subjects were the ones who came for a walk in clinic, therefore, the selection bias of illness can be present i.e., more ill subjects may be over represented.
Conclusion
From this study, we have concluded that vitamin B12 and serum folate levels significantly decreased in cases as compared to controls but there is no association between these parameters. Higher serum homocysteine levels and lower vitamin D levels are risk factor for the senile dementia of Alzheimer’s type. Further, it is concluded that after the start of the disease there is no improvement in CDR levels with supplementation of vitamin B12 and folic acid. Homocysteine is reduced by supplementing the body with vitamin B6 and vitamin B12.
Conclusion
We thank our Director, Dean and Academics Head, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India for constant support and encouragement throughout the experimental analysis and financial support.
Financial support: The study was funded by intramural fund of Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
Vitamin B
12, Folate and Vitamin D levels were not significant as compared to controls, whereas Homocysteine level was highly significant as compared to controls.
[1]. Anand R, Gill KD, Mahdi AA, Therapeutics of Alzheimer’s disease: Past, present and futureNeuropharmacology 2014 76:27-50.10.1016/j.neuropharm.2013.07.00423891641 [Google Scholar] [CrossRef] [PubMed]
[2]. Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH, Vitamin status and intake as primary determinants of homocysteinemia in an elderly populationJAMA 1993 270(22):2693-98.10.1001/jama.1993.035102200490338133587 [Google Scholar] [CrossRef] [PubMed]
[3]. Hutto BR, Folate and cobalamin in psychiatric illnessCompr Psychiat 1997 6:305-14.10.1016/S0010-440X(97)90925-1 [Google Scholar] [CrossRef]
[4]. Scott JM, Weir DG, Folic acid, homocysteine and one-carbon metabolism: a review of the essential biochemistryJ Cardiovasc Risk 1998 5:223-27.10.1097/00043798-199808000-000039919469 [Google Scholar] [CrossRef] [PubMed]
[5]. Mathieu C, Gysemans C, Giuleitti A, Bouillon R, Vitamin D and diabetesDiabetologia 2005 48(5):1247-57.10.1007/s00125-005-1802-715971062 [Google Scholar] [CrossRef] [PubMed]
[6]. Grandi NC, Breitling LP, Brenner H, Vitamin D and cardiovascular disease: systematic review and meta-analysis of prospective studiesPrev Med 2010 51(3-4):228-33.10.1016/j.ypmed.2010.06.01320600257 [Google Scholar] [CrossRef] [PubMed]
[7]. Burgaz A, Orsini N, Larsson SC, Wolk A, Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysisJ Hypertens 2011 29(4):636-45.10.1097/HJH.0b013e32834320f921191311 [Google Scholar] [CrossRef] [PubMed]
[8]. Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JL, Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare populationAm J Cardiol 2010 106(7):963-68.10.1016/j.amjcard.2010.05.02720854958 [Google Scholar] [CrossRef] [PubMed]
[9]. Grant WB, Does vitamin D reduce the risk of dementia?Journal of Alzheimers Disease 2009 17(1):151-59.10.3233/JAD-2009-10241949444 [Google Scholar] [CrossRef] [PubMed]
[10]. Pogge E, Vitamin D and Alzheimer’s disease: is there a link?Consult Pharm 2010 25(7):440-50.10.4140/TCP.n.2010.44020601349 [Google Scholar] [CrossRef] [PubMed]
[11]. McCaddon A, Kelly CL, Familial Alzheimer’s disease and vitamin B12 deficiencyAge Ageing 1994 23:334-37.10.1093/ageing/23.4.3347976784 [Google Scholar] [CrossRef] [PubMed]
[12]. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL, A new clinical scale for the staging of dementiaBr J Psychiatry 1982 140:566-72.10.1192/bjp.140.6.5667104545 [Google Scholar] [CrossRef] [PubMed]
[13]. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM, "Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease"Neurology 1984 34(7):939-44.10.1212/WNL.34.7.9396610841 [Google Scholar] [CrossRef] [PubMed]
[14]. Bates CJ, Mansoor MA, van der Pols J, Prentice A, Cole TJ, Finch S, Plasma total homocysteine in a representative sample of 972 British men and women aged 65 and overEur J Clin Nutr 1997 51:691-97.10.1038/sj.ejcn.16004689347290 [Google Scholar] [CrossRef] [PubMed]
[15]. Mattson MP, Kruman II, Duan W, Folic acid and homocysteine in age-related diseaseAgeing Research Reviews 2002 1:95-111.10.1016/S0047-6374(01)00365-7 [Google Scholar] [CrossRef]