Introduction
Medical equipments are an important and necessary component of all healthcare systems. They are introduced and utilised for patient diagnosis and treatment. These medical devices must be kept in safe condition in order to prevent injuries in patients and staff [1-3].
According to the World Health Organization, in the USA in 2010 approximately 412 US dollars were assigned to medical equipment maintenance [4]. Therefore, maintenance management system is critical to improve the reliability of medical equipment and significantly improve safety and cost-efficiency [5]. For example, proper maintenance can increase the life of equipment. Also, this process ensures us about providing appropriate health services and saving the scarce resources. However, many hospitals and healthcare organizations do not benefit from maintenance excellence [6].
Medical Equipment Management System (MEMS) includes the equipment inventory, a work order system, the preventive maintenance schedules/procedures, outsourcing contract management and all service history records. Today, the Clinical and Biomedical Engineering (CE/BME) is responsible for the healthcare asset management and healthcare technology assessment, clinical staff safety, repair and maintenance, risk and safety management, and also contrast monitoring and quality improvement [1-3]. In addition, Computerised Maintenance Management Systems (CMMS) is a fundamental information resource in most healthcare systems to track each piece of equipment and maintain accurate records of inventory and data for medical equipment [5,7,8].
There are two most common approaches for medical equipment maintenance that includes preventive and the corrective strategies. Preventative Maintenance (PM) is a scheduled process of ensuring medical devices; and the equipment are kept in perfect working conditions. Corrective Maintenance (CM) is repairing and restoration of medical devices that failed to function. These strategies help hospitals to assess or improve maintenance, quality and performance. At this point, hospitals should be considering their capability and situation to apply best maintenance systems for the large number of different medical devices in the equipment management processes [9].
Healthcare organisations should determine the criteria to assess their medical equipment maintenance management. There is a lack of specific and precise tool to assess the process of medical equipment maintenance management in Iranian hospitals. Hence, we extracted the factors affecting the medical equipment maintenance management through a systematic review method.
Materials and Methods
Data Sources and Study Selection
A systematic search of the following databases was conducted between October 11, 2015 to October 14, 2015: OVID (1860), PubMed (1966), ProQuest (1946), Scopus (1960), Embase (1974-), Science Direct (1823) and Web of Science (1983). The search was updated in June 2017. The search strategy and specific search algorithm varied based on the characteristics of each database. Each database was searched without any limitations. Since the extraction of effective factors did not depend on the type of study, there was no limit in this regard. All studies that mentioned influential factors were included. We searched databases based on title/abstract using keywords: “medical device”, “medical equipment”, and “maintenance management”
The reference lists of all retrieved studies were also searched for relevant studies that may have been missed in the database search. The initial selection of studies was performed by the first author (RB), and verified by the last author (LD). Two reviewers (RB and LD) independently screened each record for eligibility by examining titles, abstracts, and full text. In the initial screening, articles were excluded if their titles and/or abstracts were unrelated to medical equipment maintenance management. Since the extraction of effective factors did not depend on the type of study, there were no limitations in this regard. All studies mentioning influential factors were included in this review. Non-English language articles and review conferences were excluded in full text review. We contacted the corresponding author via e-mail when we could not have access to full text of articles.
Quality appraisal and data extraction
Data extraction was performed by one researcher (RB) and was recorded in a data extraction table, which includes information on study title, authors, years of study, country of study, study design, setting, aims of research, study approach, sampling, and factors affecting maintenance management, key findings and quality score. Data were abstracted by one reviewer (RB) and checked by a second reviewer (LD). In case of doubt, data were discussed until agreement was reached (RB, LD). Discrepancies were resolved using consensus. The quality of studies was assessed by two reviewers (RB and LD) using QASP checklist [10] for case studies and STROBE checklist [11] for cross-sectional studies. Studies based on earned score divided into three categories: poor, medium and good quality.
Statistical Analysis
At first, we extracted the factors, then categorised extracted factor based on Ministry of Health and Medical Education of Iran (MOHME) framework [12] in each category.
Results
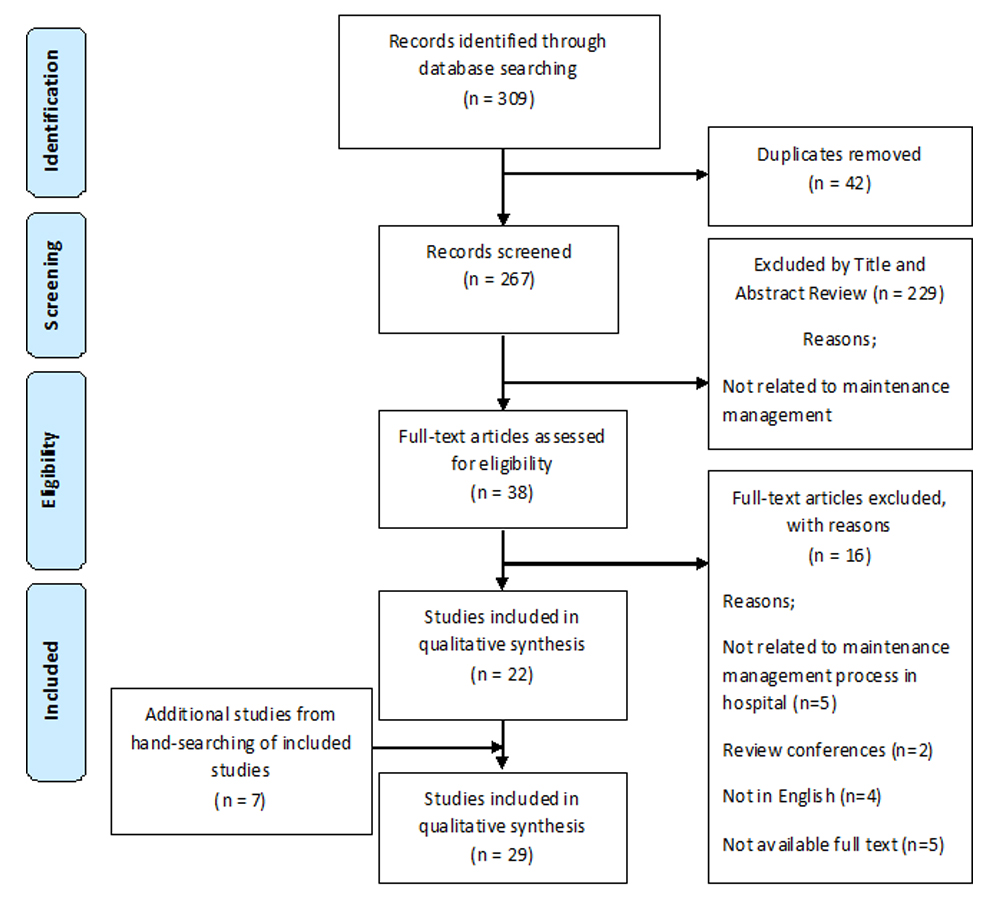
Of 309 potentially relevant articles searched, 42 papers were duplicated. The references of 22 included studies were pearled and seven additional articles were identified. Finally, a total of 29 articles were included in this review [Table/Fig-1]. At first, we extracted the factors, then, categorised extracted factor based on MOHME framework in each category. The summary characteristics of included studies are shown in [Table/Fig-2]. In terms of quality (n=14, 48.27%) studies had a good quality, (n=8, 27.58%) had medium and (n=7, 24.13%) had a poor quality
PRISMA Flow Diagram of literature search process.
Characteristics of included studies
| Study (year) | Geographic region | Study design | Setting | Quality score | Factors affecting maintenance management |
|---|
| Hamdi N et al., (2012) [5] | Jordan | Cross-sectional | Hospital | Good | Prioritizing maintenance requests: Equipment function, location of use, the load on the hospital containing the failed device, the presence of an alternative to this device in the hospital, time since maintenance request in days, and distance to the nearest hospital containing the same type of device for which maintenance is requested. Equipment quality control: Probability of failure, number of failures, DT per year, and mean service life before first failure, usability, and performance measures |
| Wang ZH (2014) [7] | China | Not reported | Hospital | Poor | Use of computer network and database information management, maintenance system lag behind, maintenance of equipment and the method of using the backward, devices lack of regular maintenance and management, medical equipment lack of details and ignorance, improper management of medical stall. |
| Al-Bashir A et al., (2012) [13] | Jordan | Case study | Hospital | Good | Short maintenance period, location and existence of more than workshop, failure response time, nature of maintenance system, safe medical device, high MTBF, training of operators on installation, periodic training of operators, calibration of maintained device, do checkup after maintenance, spare parts availability, spare parts obtaining time, doing PM, existence of redundant device, the presence of a medical engineering specialist, original spare parts. administrative procedures, enough staff, infrastructure and transportation within hospital, existence of work specialty, existence of suitable tools, enough budget, types of spare parts contracts, device strength, meet standards, continuous education and training. |
| Cohen T CN et al., (2001) [14] | California (US) | Cross-sectional | Healthcare system | Medium | CMMS, failure rate, DT, repair, MC, user/operator training, SM, inspection, work order system, aging, capital budget planning, equipment needs analysis, vendor selection, service provider evaluation, service contract evaluation, installation planning, installation, acceptance, product recalls/alerts, incident or accident investigation, replacement analysis, de-installation, salvage, maintenance requirements, equipment function, physical and clinical risks to the patient, incident history, stock parts, purchasing, warranty work |
| Masmoudi M (2016) [15] | Tunisia | Case study | Hospital | Good | CM, PM, quality control, selection and monitoring of different contracts, making recommendations for the purchasing of new devices, the training of hospital personnel, maintenance strategy, controls the rate ofequipment deterioration, ensures safety, prioritization of medical devices, maintenanceplanning, visual inspection or basicperformance check, contract monitoring, availability of resources, the possibility of training, the availabilityof tools, the cost of spare parts, and the mean time betweenfailures, complexity and frequency of failures, degree of maintenancecomplexity, function, risk, level of importance of the mission, age, detectability, frequency of failures, downtime, utilization rate, and availabilityof alternative devices |
| Chien CH et al., (2010) [16] | Taiwan | Case study | Hospital | Medium | Intranet in hospital, medical equipment management system, web server of clinical engineeringdepartment, receipt, dispatch, user retrieve, close moment, installation verification, warranty inspection, pm, contract management |
| Oshiyama NF et al., (2014) [17] | Brazil | Cross-sectional | Center for Biomedical Engineering | Good | CM data (number of events, total time and total cost), equipment age |
| Anderson JT (1992) [18] | California (US) | Review literature | Hospital | Poor | Risk of an equipment failure to patients and staff (risk coding system) |
| Kresch E KP et al., (1985) [19] | Pennsylvania (US) | Cross-sectional | Hospital | Medium | Electrical safety and functional inspection, calibration, repair, training, documentation system, preventive maintenance, acceptance |
| Baumeister J (1987) [20] | Oklahoma (US) | Case study | Hospital | Good | Maintenance expense, age equipment, equipment purchases, system design |
| Bracale M et al., (1994) [21] | Italy | Cross-sectional | Hospital | Medium | MC, trained personnel, organization and co-ordination, personnel, PM, technical documentation, training for physicians and nurses, level of technical know-how, reliable inventory of the existing equipment, information system, draft the ‘standards’ for the technology requirements |
| Cohen T (1995) [22] | California (US) | Not reported | Clinical Engineering departments | Poor | Equipment inventory, personnel records, stock parts inventory, vendor business cards, SM procedures in service manuals, data record (paper or computer), risk support capabilities, equipment control section, work orders, scheduling periodic maintenance, parts management, vendor service management, service contract management, maintenance insurance, business/employee directories, quality assurance, productivity, security, reports, utilities |
| Cram NBC et al., (1998) [23] | Texas (US) | Review | Hospital | Poor | CMMS, control and monitor equipment performance (routine performance testing, initial inspection, prevention maintenance, calibration and verification of performance, repair and action on device recalls and hazards. training programs for all users and biomedical equipment technicians. quality assurance program, equipment maintenance costs, acquisition and replacement decision, new services, planning of new construction and major renovation, risk management |
| Wang B and Levenson A (2000) [24] | Staten Island (US) | Not reported | Hospital | Medium | Inspection, user training, equipment aging, severity failures, equipment function, physical risk |
| Kaur R et al., (2005) [25] | Hartford (US) | Case study | Hospital | Good | MEMP:inspection, PM and education of equipment users and maintainers |
| Wang B et al., (2006) [26] | California (US) | Not reported | Hospital | Poor | SM activities, safety and performance inspection, documentation, user training, failure, calibration, repair and replacement |
| Kullolli I (2008) [27] | Boston (US) | Not reported | Hospital | Poor | CMMS, PM, training, maintenance history, downtime of a device, parts inventory, purchase orders |
| Tarawneh W et al., (2009) [28] | Jordan | Cross sectional | Hospital | Good | MEC: Safety, working performance, age, maintenance cost, utilization, up time or down time, operational cost, income value, possibility for upgrading, availability of repair parts, availability of new technology. Medical equipment DT: Availability of spare parts, type of failures accrued, qualification level and experience of biomedical engineers and technicians, equipment priority for servicing, the utilization level and operational load, the working environment condition, the equipment technology level. MC: Cost of spare parts used during corrective and PM, cost of accessories replaced, BMEs labor (work + transportation) cost, all administrative and logistic cost to allocate parts and services, upgrading and modification costs, a training costs. Factors that impact maintenance cost of medical equipment are: Spare parts cost, type of failures accrued, maintenance level used (component/board/ module level), labor (man Power + transport) cost, maintenance tool kits and test equipment, routine and planned preventive Maintenance cost |
| Youssef NF et al., (2009) [29] | Texas (US) | Cross sectional | Hospital | Medium | Equipment maintainability, installation, repair and connectivity |
| Wang BSCCE et al., (2010) [30] | North Carolina (US) | Cross sectional | Hospital | Good | Unscheduled activities: consist of repairs (or CM) and replacement SM: (SPI), monitoring parameters to predict failures (predictive maintenance) and preventing future failures (PM) |
| Wang BSCCE et al., (2010) [31] | North Carolina (US) | Cross sectional | Hospital | Good | Maintenance Activities Included: Equipment Acceptance, Operator Check, Safety and Performance Inspection, PM, Repair Replacement. CM, SM |
| Alves ET et al., (2011) [32] | Brazil | Case study | Clinical engineering company | Good | Types of maintenance: corrective, preventive, calibration and technical analysis MMS: Automatic generation of preventives orders, gradual reduction in corrective maintenances, gradual reduction in correctives into hospital, inventory control, budget management, remote monitoring and qualification.MMS several direct benefits: Time, reduction human error, reduction of cm, inventory control, traceability, quality assessment |
| Taghipour S et al., (2011) [33] | Canada | Cross sectional | Hospital | Good | RCM,PEMP: Inspection, PM, and testing of medical equipment, classification of medical devices, guidelines Prioritization (Function, Mission criticality, Utilization, Availability of alternative devices, Age, Risk, Failure frequency, Detectability, Failure consequence, Operational, DT, Non-operational, Cost of repair, Safety and environment, Recalls and hazard alerts, Maintenance requirements), maintenance strategies |
| Taghipour S (2011) [34] | Canada | Cross sectional | Hospital | Good | Performance, risk, resource inputs and cost, MEMP, PM: Acceptance Test, operational check, safety and performance inspection, failure preventive actions: replacement, calibration, lubrication CMMS Core Modules: Inventory control: Work order management system, scheduling/ planning, vendor management, parts management, PM, labor, purchasing, budgeting prioritization of medical devices, field maintenance data, trend test, inspection interval, remarks |
| Wang BSCCE et al., (2011) [35] | North Carolina (US) | Cross sectional | Hospital | Good | CM, SM, user training, purchase assistance, and acquisition planning |
| Mokfi T et al, (2011) [36] | Iran | Case study | Hospital | Good | Prioritizing medical equipment, suggesting appropriate PM scheduling, manufacturer data, Failure data, PM data (MTBF, MTTF), experts, priority and risk factors, resultant data, prominent guidance, international standards |
| Kinley CA (2012) [37] | East Tennessee (US) | Not reported | Hospital | Medium | Electrical safety checks, calibrating, test equipment, PM (parts, cleaning, lubricating, PM sticker) repairs(priority and no priority), incoming inspections, functional checks, evidenced based planning, risk-based planning, equipment history, hospital intranet system, MEMP, maintenance plan, documentation CMMS database (Hospital Equipment ID, Equipment description, device type, manufacturer, model, serial number, assigned technician, SM information, department account, location, risk category, device category, vendor, purchase order number, purchase cost, installation Date: date entered into service, warranty start and end dates) trained staff, contracts, IT |
| Wang JK (2014) [38] | China | Cross sectional | Hospital | Poor | Management system lags behind, lack of regular maintenance and management, maintenance methods out of date, medical engineering technicians serious erosion, the selection of instruments and equipment parameter, acceptance of the instrument, staff training, management records, implement scientific management method, levels of education, skills and narrow scope in personnel |
| Ibey AAMMPCCE et al., (2015) [39] | Canada | Cross sectional | Clinical engineering department | Medium | Aspects of a CMMS: Assets, work orders, internal and external service records, PM schedules, warranty periods, parts inventory, service contract and purchasing information, quality assurance, reporting, cost control, and alerts and hazards |
MTBF: Mean Time Between Failures, PM: Preventive Maintenance, CMMS: Computerized Maintenance Management Systems, MEMS: Medical Equipment Management System, CED: Clinical Engineering Department, DT: Down Time, MC: Maintenance Cost, BMEs: Biomedical Engineers, CM: Corrective Maintenance, SM: Scheduled Maintenance, MMS: Maintenance Management System, RCM: Reliability Centered Maintenance, MEMP: Medical Equipment Management Program, MTTF: Mean Time To Failures, MEC: Medical Equipment Condition
All of the identified studies implied at least one of the items and factors affecting medical equipment maintenance management process. Fifteen of the included studies were from the US, while the remaining studies were from other countries (Jordan=3, Canada=3, China=2, Brazil=2, Tunisia, Italy, Taiwan and Iran=1). Of the 29 studies identified, 7 (24.13%) were case study, 14 (48.27%) cross sectional, 2 (6.89%) literature review and 20.68% of them did not report study design. All included studies except one of them was conducted in hospital setting. The three studies including Cohen and Cram (2001), Al-Bashir A et al., (2012) and Masmoudi M et al., (2016) reported the highest number of factors [13-15]. In total, 12 studies cited to user training, 17 to PM, nine to record or documentation and six to CMMS.
The factors extracted from these studies were classified based on information contained in the medical equipment maintenance management criteria of the MOHME [12], including management, resources, service, inspection, quality control, information bank and education. We identified 89 factors repeatedly mentioned in the literature as important determinants of medical equipment maintenance management process. We found five of the factors were significantly related to resources item, 12 related to service, four contains education, 15 of these regarding quality control, 19 related to inspection, 12 related information bank and 22 were dedicated to management factor [Table/Fig-3].
Categorisation of factors affecting medical equipment maintenance management process based on Iranian MoH criteria.
| Items | Factors affecting the medical equipment maintenance management |
|---|
| Management | Equipment purchases System design Organization and coordination Equipment control section Parts management Vendor service management Service contract management Maintenance insurance Business/employee directories Prioritizing maintenance requests Control and monitor equipment Performance
| Planning of new Construction and major renovation MEMP RCM Predictive maintenance PM CM SM Acquisition planning Work order management system Evidenced based planning Risk-based planning
|
| Resources | Existence of work specialty Enough staff Existence of administrative person
| Using assets Enough budget |
| Information bank | CMMS Prominent guidance, international Standards Manufacturer data Equipment history Failure data Hospital intranet system Documentation
| IT Computer network and Database information Management Internal and external Service records Service contract and Purchasing information Reporting
|
| Service | Age equipment MC Work orders Service contract management Acquisition and replacement decision Medical equipment DT
| Repairs and replacement Work order management system Failure data Failure response time High MTBF Types of spare parts Contracts
|
| Inspection | Equipment and stock parts inventory Medical equipment condition Equipment acceptance Operator check Installation verification Warranty inspection Safety and performance inspection Incoming inspections Spare parts availability Functional checks
| Location and existence of More than workshop Spare parts obtaining time Cost control Existence of redundant Device Original spare parts Administrative procedures Infrastructure & Transportation within Hospital Existence of suitable tools Productivity, utilities
|
| Education | Training of operators on installation Trained staff | Training programs Level of technical know-How |
| Quality control | Test equipment Acceptance test Technical inspection (safety, Operational and calibration checks) Risk support capabilities Quality assurance program Security Risk management
| SPI Equipment quality control Calibration and technical Analysis Risk factors Electrical safety checks Safe medical device Do checkup after Maintenance Alerts and hazards
|
MTBF: Mean Time Between Failures, PM: Preventive Maintenance, CMMS; Computerized Maintenance Management Systems, DT: Down Time, MC: Maintenance Cost, CM: Corrective Maintenance, SM: Scheduled Maintenance, RCM: Reliability Centered Maintenance, MEMP: Medical Equipment Management Program
Discussion
This study has identified and extracted all the factors and elements related to the maintenance management and have created a comprehensive view. Twenty-nine studies met the inclusion criteria for this systematic review; however, each of the studies investigated different aspects of the process for medical equipment maintenance management. In other words, the purpose of each of the study was different. All the studies except one of them was conducted in hospital. Most 14 (48.2%) studies used cross sectional design. The methodological quality of the papers studied was variable. This review has evaluated a limited number of studies because the number of studies directly referring to this issue were limited. Although the review has identified several important factors, they should be seen as maintenance management framework.
Based on our study results, we conducted a new classification for medical equipment maintenance management that contains all activities and the factors affecting it. This classification has seven categories which include: management, information bank, inspection, quality control, education, resources and service. These categories is in relation to issues such as medical equipment management cycle that starts from the purchase of equipment to management risk, management of spare parts and maintenance priorities, as well as includes a variety of maintenance strategies.
Significant factor in the management category was medical equipment maintenance programs that include predictive maintenance, preventive maintenance, corrective maintenance and scheduled maintenance. In the resource category, the importance of human resources has been mentioned repeatedly. Significant factor in the service category was contract of maintenance. Trained staff was another important factor in maintenance of medical equipment. Documentation and CMMS were two important factors in the information bank. Safety and performance inspection and calibration were important components of quality control.
Information bank refers to data collection, reporting, CMMS, service records and IT. The inspection is associated with safety and performance inspection, spare parts availability, cost control, infrastructure, administrative procedures and productivity, utilities. Acceptance test, quality assurance program, security, calibration and technical analysis, risk factors, electrical safety checks, safe medical device and alerts and hazards are in quality control. Education refers to training programs, trained staff and level of technical know-how. The sources include existence of work specialty, enough staff, assets and enough budgets. Eventually, service items include age equipment, maintenance cost, work Conditionorders, service contract management, down time, repairs and replacement, failure data and acquisition and replacement decision.
According to studies carried out by Cohen T CN et al., (2001), Kresch E KP et al., (1985), Cohen T (1995), Cram NBC et al., (1998), Kullolli (2008) and Ibey AAMMPCCE et al., (2015) it was determined that the computerized maintenance management system is one of the important and effective factors in the implementation maintenance management[14,19,22,23,27,39]. Studies showed that the procurement of computerised maintenance management system should be the goal of all clinical engineering departments.Indeed, CMMS implies the importance of documenting and reporting requirements related to maintenance management process.
According to the findings, in the medical equipment maintenance procedures, the PM is mentioned repeatedly among the factors affecting maintenance management process. PM is one of the main functions of the biomedical engineering department. Nearly half of the studies indicated that doing preventive maintenance was necessary for most medical devices [13,15,16,21,25,27,28,30,31,33,34,36,37,39].
According to our findings, user training is one of the main components of maintenance management. Many studies have mentioned the importance of existence of trained force and having a training program for physicians and other users. Factors related to user training were frequently mentioned in most studies [13-15,19,21,23,24,27,29,35,37,38].
SM is one of the maintenance activities that consist of SPI, PM and predictive maintenance. Approximately most studies have referred to one of these [14,22,26,30,31,37].
Unscheduled activities consist of repairs or (CM) and replacement that cannot be planned and scheduled. According to studies CM activities required for all kinds of medical equipment [15,17,30-32,35]. This is consistent with our findings.
As our findings also indicated, three studies mentioned a clinical engineering department needs to create and maintain a MEMP [25,33,37]. This document will outline all policies and procedures required. This plan includes acquisition planning, evidenced based planning and risk-based planning.
Documentation is one of the important factors in maintenance management, which studies point to the importance of this factor [19,21,26,37]. Proper documentation is necessary for a successful biomedical engineering department. Equipment history, manufacturer data, failure data, service contract and purchasing information must be clearly documented.
Having enough staff is a must for any biomedical engineering department to be successful which was confirmed by our findings. As well as Al-Bashir A et al., (2012) considers providing sufficient staff to be the basis for maintenance management [13].
According to the findings, maintenance cost is a critical factor that should be taken into consideration when deciding to replace medical equipment [15,17,23,28,33,34,37,39]. MC include all maintenance costs such as cost of spare parts used during corrective and PM, cost of accessories replaced, BMEs labor (work + transportation) cost, all administrative and logistic cost to allocate parts and services, upgrading and modification costs and training costs.
The study of Chien CH et al., showed that maintenance practices are one of the medical equipment management sub-systems which is associated with other sub-systems, i.e., the basic information, procurement, acceptance, discard, installation verification, warranty inspection, prevent maintenance, and contract management[16]. Also, another study that was conducted by Youssef NF and Hyman WA, in Texas, cited to maintenance management elements such as parts, install, health record system, equipment selection and acquisition, equipment maintenance programs, training programs for clinical users and biomedical technicians, and risk management, performance testing, inspection, preventive maintenance, repair, equipment classified, control and monitoring of equipment performance [29]. Many studies have pointed to a variety of maintenance strategies. In fact, maintenance activities have different types: corrective maintenance, preventive maintenance, quality control, selection and monitoring of different contracts with suppliers, subcontractors, and service companies and making recommendations for the purchasing of new devices and the training of hospital personnel.
Medical equipment maintenance management includes equipment age, physical risk to patients, maintenance requirements, availability of maintenance tools, and availability of competent staff, warranty periods, parts inventory and other factors. In addition, some factors were overlapping with each other. For example, some of the factors related to quality control item were in common with the item inspection, such as: acceptance test, Safety and Performance Inspection (SPI) and functional checks. A number of factors related to management section overlap with service item, such as: work order management system, service contract management, types of spare parts contracts, vendor service management, acquisition and replacement decision. Factors affecting medical equipment maintenance management are important in priority equipment for the purchase, repair and maintenance and also in a cost effective hospital management. Thus, it can be concluded that all seven items of medical equipment maintenance management relate to each other and are interdependent. The present systematic review extracted the influential factors from studies that are related to maintenance management context but similar studies need to be done. Therefore, there is a need for studies in this field because the other factors should be identified and evaluated.
Limitation
One of the most important limitation is lack of fluency in other languages to use the results of Non-English language studies. Perhaps, more factors could be identified if the results of Non-English studies could be added. Lack of access to full-texts of some article was also another limitation.
Conclusion
This systematic review of the literature has found evidence that human resource training, medical equipment maintenance programs, contract of maintenance, safety and performance inspection and calibration, documentation and CMMS are critical factors in medical equipment maintenance management. This review has also introduced a certain classification for medical equipment maintenance management, which in fact has provided some useful information to provide a framework as an asset to this process. All the items involved in the process of assessing the status of maintenance of medical equipment has to be considered in order to make the right decisions for future operations.
We conclude that considering a maintenance management process brings direct benefits to a medical engineering sector. The most significant finding of this review is the need for further research in the field of maintenance of medical devices, as indicated by the gaps in existing research detailed above.
MTBF: Mean Time Between Failures, PM: Preventive Maintenance, CMMS: Computerized Maintenance Management Systems, MEMS: Medical Equipment Management System, CED: Clinical Engineering Department, DT: Down Time, MC: Maintenance Cost, BMEs: Biomedical Engineers, CM: Corrective Maintenance, SM: Scheduled Maintenance, MMS: Maintenance Management System, RCM: Reliability Centered Maintenance, MEMP: Medical Equipment Management Program, MTTF: Mean Time To Failures, MEC: Medical Equipment Condition
MTBF: Mean Time Between Failures, PM: Preventive Maintenance, CMMS; Computerized Maintenance Management Systems, DT: Down Time, MC: Maintenance Cost, CM: Corrective Maintenance, SM: Scheduled Maintenance, RCM: Reliability Centered Maintenance, MEMP: Medical Equipment Management Program
[1]. Lenel A, Temple-Bird C, Kawohl W, Kaur M, How to organize a system of healthcare technology management. Geneva: World Health Organization 2005 10.1049/ic.2006.0660 [Google Scholar] [CrossRef]
[2]. David Y, Jahnke EG, Planning hospital medical technology managementIEEE Eng Med Biol Mag 2004 23:73-79.10.1109/MEMB.2004.131798515354998 [Google Scholar] [CrossRef] [PubMed]
[3]. David Y, Judd TM, Management and Assessment of Medical Technology, Clinical Engineering (Principles and Applications in Engineering) 2003 New YorkCRC10.1201/9780203009178.ch2 [Google Scholar] [CrossRef]
[4]. Pammolli F, Riccaboni M, Oglialoro C, Magazzini L, Baio G, Salerno N, Medical devices competitiveness and impact on public health expenditure 2005 [Google Scholar]
[5]. Hamdi N, Oweis R, Abu Zraiq H, Abu Sammour D, An intelligent healthcare management system: a new approach in work-order prioritization for medical equipment maintenance requestsJ Med Syst 2012 36(2):557-67.10.1007/s10916-010-9501-420703695 [Google Scholar] [CrossRef] [PubMed]
[6]. Ginsburg G, Human factors engineering: A tool for medical device evaluation in hospital procurement decision-makingJ. Biomed. Inform 2005 38(3):213-19.10.1016/j.jbi.2004.11.00815896694 [Google Scholar] [CrossRef] [PubMed]
[7]. Wang ZH, Application Research on the Internet in the Management of Medical Equipment Maintenance. Applied Mechanics and MaterialsTrans Tech Publ 2014 651:1535-1538.10.4028/www.scientific.net/AMM.651-653.1535 [Google Scholar] [CrossRef]
[8]. Basiony M, Computerized equipment management systemJ Clin Eng 2013 38:178-84.10.1097/JCE.0b013e3182a904e4 [Google Scholar] [CrossRef]
[9]. The Joint Commission. Comprehensive Accreditation Manual for Hospitals.2010. [Available from: https://www.jointcommission.org ] [Google Scholar]
[10]. CASP Tools Checklist. http://www.casp-uk.net/casp-tools-checklists 2016/5/2 [Google Scholar]
[11]. STROBE Checklist. In: Strobe-statement.org, editor. https://www.strobe-statement.org/index.php?id=available-checklists Access date: 2016/5/2 [Google Scholar]
[12]. Medical equipment maintenance management criteria of the Ministry of Health and Medical Education of Iran [Available at] http://www.fda.gov.ir/uploads/59654f7fc6cbc314a8be0776c15f08fe.pdf 2015/8/28 [Google Scholar]
[13]. Al-Bashir A, Al-Rawashdeh M, Al-Hadithi R, Al-Ghandoor A, Barghash M, Building medical devices maintenance system through quality function deploymentJordan J. Mech. Ind. Eng 2012 6(1):25-36. [Google Scholar]
[14]. Cohen T, Cram N, Computerized maintenance management systemsJ Clin Eng 2001 26(3):200-11.10.1097/00004669-200126030-00007 [Google Scholar] [CrossRef]
[15]. Masmoudi M, Houria ZB, Hanbali A, Masmoudi F, Decision Support procedure for medical equipment maintenance managementJ Clin Eng 2016 41(1):19-29.10.1097/JCE.0000000000000135 [Google Scholar] [CrossRef]
[16]. Chien CH, Huang YY, Chong FC, A framework of medical equipment management system for in-house clinical engineering department. 2010 32nd Annual International Conference of the IEEE Engineering in Medicine and Biology SocietyEMB’10 2010 [Google Scholar]
[17]. Oshiyama NF, Silveira AC, Bassani AK, Bassani JWM, Medical equipment classification according to corrective maintenance data: A strategy based on the equipment ageRev. Bras. Eng. Biomed 2014 30(1):64-69.10.4322/rbeb.2013.045 [Google Scholar] [CrossRef]
[18]. Anderson JT, A risk-related preventive maintenance systemJ Clin Eng 1992 17(1):65-68.10.1097/00004669-199201000-0002310117005 [Google Scholar] [CrossRef] [PubMed]
[19]. Kresch E KP, Schwartz H, Hamarman H, A computerized hospital maintenance systemJ Clin Eng 1985 10(1):13-22.10.1097/00004669-198501000-0000410271514 [Google Scholar] [CrossRef] [PubMed]
[20]. Baumeister J, Hospital financial audit of medical equipment maintenance: A case studyJ Clin Eng 1987 12(3):197-201.10.1097/00004669-198705000-0001010282302 [Google Scholar] [CrossRef] [PubMed]
[21]. Bracale M, Pepino A, Medical technologies in developing countries: a feasibility study on the maintenance of medical equipment in EthiopiaMed. Biol. Eng. Comput 1994 32(2):131-37.10.1007/BF025189098022208 [Google Scholar] [CrossRef] [PubMed]
[22]. Cohen T, Computerized maintenance management systems: How to match your department’s needs with commercially available productsJ Clin Eng 1995 20(6):457-68.10.1097/00004669-199511000-0000810152788 [Google Scholar] [CrossRef] [PubMed]
[23]. Cram NBC, Computerized Maintenance Management Systems: A Review of Available ProductsJ Clin Eng 1998 23(3):169-79.10.1097/00004669-199805000-00016 [Google Scholar] [CrossRef]
[24]. Wang B, Levenson A, Equipment inclusion criteria-a new interpretation of JCAHO’s medical equipment management standardJ Clin Eng 2000 25(1):26-35.10.1097/00004669-200025010-00009 [Google Scholar] [CrossRef]
[25]. Kaur R, Defrancesco V, Enderle J, Design, development and evaluation of an Asset Tracking System. Proceedings of the 2005 IEEE 31st Annual Northeast Bioengineering Conference 2005 NJHoboken [Google Scholar]
[26]. Wang B, Furst E, Cohen T, Keil OR, Ridgway M, Stiefel R, Medical equipment management strategies. BiomedInstrum. Technol 2006 40(3):233-37.10.2345/i0899-8205-40-3-233.116796335 [Google Scholar] [CrossRef] [PubMed]
[27]. Kullolli I, Selecting a computerized maintenance management systemBiomed Instrum Technol 2008 42(4):276-78.10.2345/0899-8205(2008)42[276:SACMMS]2.0.CO;2 [Google Scholar] [CrossRef]
[28]. Tarawneh W, El-Sharo S, Assessment of medical equipment in respect [28]to their down time. World Congress on Medical Physics and Biomedical Engineering: Diagnostic and Therapeutic InstrumentationJ Clin Eng 2009 Munich10.1007/978-3-642-03885-3_74 [Google Scholar] [CrossRef]
[29]. Youssef NF, Hyman W. A., A medical device complexity model: a new approach to medical equipment managementJ Clin Eng 2009 34(2):94-98.10.1097/JCE.0b013e31819fd711 [Google Scholar] [CrossRef]
[30]. Wang BSCCE, Fedele JAASC, Pridgen B, Williams AAASC, Rui TSI, Barnett LA, Evidence-Based Maintenance: Part I: Measuring Maintenance Effectiveness With Failure CodesJ Clin Eng 2010 35(3):132-44.10.1097/JCE.0b013e3181e6231e [Google Scholar] [CrossRef]
[31]. Wang BSCCE, Fedele JAASC, Pridgen B, Williams AAASC, Rui TSI, Barnett LA, Evidence-Based Maintenance: Part II: Comparing Maintenance Strategies Using Failure CodesJ Clin Eng October/December 2010 35(4):223-30.10.1097/JCE.0b013e3181f6b80b [Google Scholar] [CrossRef]
[32]. Alves ET, De Oliveira LLB, Direct benefits of a Maintenance Management System: A case study. Pan American Health Care Exchanges, PAHCE 2011- Conference, Workshops, and Exhibits. Cooperation/Linkages: An Independent Forum for Patient Care and Technology Support 2011 Rio de Janeiro [Google Scholar]
[33]. Taghipour S, Banjevic D, Jardine AKS, Prioritization of medical equipment for maintenance decisionsJ. Oper Res Soc 2011 62(9):1666-87.10.1057/jors.2010.106 [Google Scholar] [CrossRef]
[34]. Taghipour S, Reliability and maintenance of medical devices [Ph.D.]Ann Arbor: University of Toronto (Canada) 2011 88(1):547-52.[Available from: http://search.proquest.com/docview/911784341?accountid=41307 ] [Google Scholar]
[35]. Wang BSCCE, Fedele JAASC, Pridgen B, Williams AAASC, Rui TSI, Barnett LA, Evidence-Based Maintenance: Part III, Enhancing Patient Safety Using Failure Code AnalysisJ Clin Eng 2011 36(2):72-84.10.1097/JCE.0b013e318214313c [Google Scholar] [CrossRef]
[36]. Mokfi T, Almaeenejad M, Sedighi MM, A data mining based algorithmto enhance maintenance management: A medical equipment case study. 1st International Conference on Informatics and Computational Intelligence, ICI 2011 Bandung. [Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84857176192&partnerID=40&md5=1740741eec3081ee11b016af02f2ce93 } [Google Scholar]
[37]. Kinley CA, Healthcare Technology: A Strategic Approach to Medical Device Management [M.S.]. Ann Arbor: East Tennessee State University; 2012 [Available from: http://search.proquest.com/docview/1019056172?accountid=41307 ] [Google Scholar]
[38]. Wang JK, Management and maintenance on CT, MRI, Gamma-ray Radiotherapy instrument. In: Liu HW, Wang G, Zhang GW, editors. 3rd International Conference on Advanced Engineering Materials and Architecture Science, ICAEMAS 2014 Trans Tech Publications Ltd:1599-602.10.4028/www.scientific.net/AMM.651-653.1599 [Google Scholar] [CrossRef]
[39]. Ibey AAMMPCCE, King DMP, Hsieh TBP, Hutnan TB, Dixon JB, Soet RB, What Constitutes a Clinical Engineering Asset?J Clin Eng 2015 40(3):165-68.10.1097/JCE.0000000000000103 [Google Scholar] [CrossRef]