Introduction
The increased activity of enzyme ALDR-2 has been recognised as a major risk factor for the development of microvascular complications in diabetic patients[1-3] that comprises neuropathy, nephropathy and retinopathy. Among these microvascular complications, DR is different in having two-staged disease progression. DR initially develops as NPDR due to the increased activity of ALDR-2. If NPDR remains uncontrolled, it later progresses to PDR that has a multifactorial causation resulting in active angiogenesis in the retina [4].
Proliferative diabetic retinopathy is a serious diabetic microvascular complication that affects the quality of life by contributing to the visual loss in diabetic patients. The pathological importance of PDR lies in the fact that it presents as a sudden diminution or complete loss of vision; whereas, the development and manifestation of the other two microvascular complications involves a relatively long time course [5]. Neovascularization in PDR results in the formation of delicate capillaries over the retina, which ruptures easily by a sudden change in the blood pressure, leading to the haziness of vitreous humour due to haemorrhage and may cause immediate blindness.
Although, the age of the patient [6], the duration of diabetes, higher glycosylated haemoglobin levels, the presence of proteinuria and hypertension have been established as risk factors for DR [7], the current knowledge about other causes like deranged lipid profile remains unsatisfactory. Polyol pathway initiation is the foundation unit for production of sorbitol, fructose and other harmful by products in PDR that leads to various alterations in the intracellular and the extracellular environment of cells [8]. These changes include: modification of intracellular tonicity, due to the generation of ‘Advanced Glycation End Products’ (AGEs); and exposure of cells to oxidative stress presumably through the production of oxidant species coupled with declining antioxidant defence mechanism [9].
Apart from polyol pathway, other pathways like Protein Kinase C (PKC) activation, glucose autoxidation, prostanoid synthesis, protein glycation and hexosamine pathway might play a minor role in the establishment of DR [10,11]. These pathways ultimately result in the degeneration of pericyte cells by apoptosis that leads to the damage of blood-retinal barrier causing haemorrhage along with the leakage of lipid and protein macromolecules present in plasma [12]. In the highly oxidative environmental condition of the retina, these lipids and modified protein will cause accumulation of ‘advanced lipoxidation end products’ [13]. Thus, DR progresses to PDR, manifesting the signs of the disease.
Several studies have been done to correlate the increased activity of ALDR-2 with various traditional and non-traditional lipid markers for the establishment of DR [14]. However, to the best our knowledge, till date none of the studies have clarified the role of dyslipidemia in the progression of DR. We hypothesised that both, dyslipidemia and increased ALDR-2 activity are responsible for the progression of NPDR to PDR. The aim of the current study was to determine whether dyslipidemia or increased ALDR-2 activity plays a major role in the progression of DR from NPDR to PDR.
Materials and Methods
The present cross-sectional was conducted during 2016-2017 that involved 200 subjects, of which, 50 subjects were serving as healthy controls (NDNR) while rest 150 had Type 2 DM. Subjects having Type 2 DM were further divided into three equal groups after ophthalmoscopy examination into those having diabetes with no retinopathy (DNR, n=50), NPDR (n=50) and PDR (n=50). Group and subgroup wise distribution is shown in [Table/Fig-1].
Group and subgroup wise distribution of subjects.
| Group | Sub group | Description | No. of patients |
|---|
| Study group (Type 2 DM) | 1 | Diabetes mellitus with Non-Proliferative Diabetic Retinopathy (NPDR) | 50 |
| 2 | Diabetes mellitus with Proliferative Diabetic Retinopathy (PDR) | 50 |
| 3 | Diabetics mellitus with No Retinopathy (DNR) | 50 |
| Control group | 4 | No Diabetes and No Retinopathy (NDNR) | 50 |
According to WHO (2015 data) prevalence of diabetes in India was 8.7% [15]. From the above data p-value for diabetics is 0.087, at 5% margin of error and 95% confidence interval, the total sample size calculated was 122. However, we included 150 diabetic patients and 50 normal subjects in our study.
The present study was performed at the Retina Clinic of Ophthalmology Department in collaboration with the Physiology Department, King George’s Medical University, Lucknow; after obtaining ethical clearance from the Institutional Ethical Committee. Subjects were recruited after a thorough history and blood sugar test. Before inclusion of diabetic patients in the study, they had their fundus examined by both direct and indirect ophthalmoscopy for the demarcation and categorization of DR according to (ETDRS) (Early Treatment Diabetic Retinopathy Study) classification [16].
Exclusion criteria were the diabetics on insulin or statin treatment and those who were anaemic or had any liver disease. A complete record of each participant, about their age, gender, clinical symptoms, duration of diabetes, medication and the socioeconomic background was noted using a well-designed questionnaire. All individuals gave the written informed consent before involvement in the study as subjects.
A fasting blood sample was drawn in the morning at 8-9 AM from the subjects into the Ethylene Diamine Tetra Acetic acid (EDTA), sodium fluoride (NaF), and plain vial, for ALDR-2, glucose and lipid profile estimation respectively. All the tests were executed on the same day of sampling in the clinical laboratory of Biochemistry Department, KGMU, Lucknow.
A 10 % erythrocyte suspension was made by adding 50 mM sodium phosphate buffer, pH 7.4, containing 150 mM NaCl. The cells in the suspension were lysed by repeated freezing and thawing (three cycles) and then centrifuged to remove any insoluble material. ALDR-2 activity was measured spectrophotometrically using an appropriately diluted haemolysate according to a previously described method [17] using a SpectraMax spectrophotometer (Molecular Devices, Sunnyvale, CA).
A total of 5 parameters: Triglyceride (TG), Total Cholesterol (TC), High Density Lipoprotein (HDL), LDL and Very Low Density Lipoprotein (VLDL) were assessed by auto-analyser.
Glucose was estimated in plasma by the Glucose Oxidase-Peroxidase (GOD-POD) method using a kit. 10 microlitres of serum or standard (100 mg/dL glucose) was added to a reagent (100 mM phosphate buffer, pH 7.5 with 5 mM phenol, 10 U/mL glucose oxidase, 1 U/mL peroxidase and 0.4 mM 4-amino antipyrine) and incubated for 5 minutes at 37°C. Absorbance was measured at 505 nm by an auto-analyser.
Statistical Analysis
The results are presented in Mean±SD. The Chi-square test was applied to compare the age between groups and subgroups. The unpaired t-test was applied to compare the continuous study parameters between two groups and subgroups. The p-value<0.05 was considered significant. All the analysis were carried out using IBM SPSS Statistics for Windows, version 22.0 (Armonk, NY: IBM Corp).
Results
Outcomes of current study based on mean age, fasting blood glucose level, ALDR-2 enzyme level, and various traditional lipid markers with respect to NDNR, DNR, NPDR and PDR subgroups are summarised in [Table/Fig-2].
Showing comparison for variable on the basis of mean, number of subjects (n) and standard deviation between NDNR, DNR, NPDR and PDR subgroups.
| Parameters | Control | Study Group | Unpaired t-test (p-value) |
|---|
| NDNR (N=50) | DNR (N=50) | NPDR (N=50) | PDR (N=50) | NDNR vs DNR | DNR vs NPDR | NPDR vs PDR |
|---|
| Age (years) | 55.84±5.62 | 56.02±7.93 | 56.92±7.22 | 57.50±6.95 | 0.8961 | 0.5543 | 0.6833 |
| FBS (mg/dL) | 97.00±26.46 | 132.68±51.13 | 137.28±56.17 | 141.73±38.54 | 0.001* | 0.6694 | 0.47 |
| PPS (mg/dL) | 134.64±71.53 | 210.08±88.96 | 192.32±79.18 | 224.65±58.03 | 0.001* | 0.2943 | 0.48 |
| TG (mg/dL) | 153.04±61.37 | 161.08±47.78 | 168.35±92.22 | 190.36±80.57 | 0.4665 | 0.6217 | 0.2080 |
| TC (mg/dL) | 148.80±23.77 | 155.38±38.39 | 168.36±41.70 | 168.40±41.08 | 0.3053 | 0.1011 | 0.1182 |
| HDL-C (mg/dL) | 51.24±11.87 | 44.60±16.28 | 43.42±10.62 | 42.40±10.64 | 0.0218* | 0.4685 | 0.6390 |
| LDL-C (mg/dL) | 63.92±19.52 | 78.77±29.61 | 84.60±40.66 | 101.60±32.82 | 0.0038* | 0.4143 | 0.0235* |
| VLDL (mg/dL) | 31.60±12.58 | 31.93±8.72 | 35.50±17.5 | 38.40±16.38 | 0.8791 | 0.2015 | 0.3914 |
| ALDR-2 (unit/gm of Hb) | 3.56±1.68 | 4.24±2.11 | 7.47±2.02 | 8.22±2.19 | 0.0777 | 0.0001* | 0.20 |
*significant, NDNR; No diabetes and no retinopathy, DNR; Diabetic but no retinopathy, NPDR; Non-proliferative diabetic retinopathy, PDR; Proliferative diabetic retinopathy, FBS; Fasting blood sugar, PPS; Post prandial sugar, TG; Triglyceride, TC; Total cholesterol, HDL-C; High density lipoprotein cholesterol, LDL-C; Low density lipoprotein cholesterol, VLDL; Very low density lipoprotein, ALDR; Aldose reductase.
In DNR group fasting and postprandial blood sugar along with LDL-C and HDL-C were significantly deranged, as compared to NDNR group. However, ALDR-2 was non-significantly increased in DNR than NDNR. A significantly higher activity of ALDR-2 was observed for NPDR subjects as compared to DNR subjects (p-value <0.001) as evident in [Table/Fig-2].
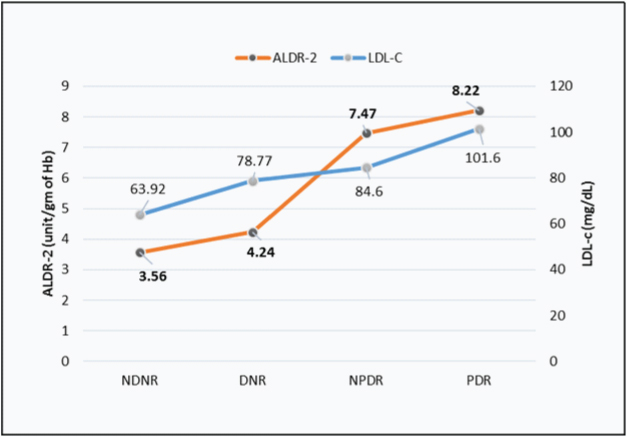
[Table/Fig-3] shows a higher value of LDL-C and ALDR-2 in NPDR and PDR group than DNR and NDNR group. However, PDR group showed a non-significant increase in ALDR-2 level while a significantly higher level of LDL-C as compared to NPDR group.
Line diagram for ALDR-2 shows a significant rise of the slope between DNR to NPDR, whereas for LDL-C significant rise observed between NPDR to PDR.
Discussion
The present study conducted in diabetic patients to know the role of dyslipidemia in the progression of NPDR to PDR. The most likely reason for a significantly higher activity of ALDR-2 in NPDR than DNR subjects could be the activation of ALDR precursor gene in diabetics causing retinopathy [18]. However, the difference in ALDR-2 level in NPDR and PDR subjects was non-significant; thus, the ALDR-2 activity might not play a major role in the progression of NPDR to PDR. Further, out of the five traditional lipid markers in our study, LDL-C was significantly high in PDR than NPDR group signifying the role of dyslipidemia in the progression of DR.
Type 2 Diabetes occurs due to insulin resistance i.e., secretion of insulin is either normal or increased, however, this increased insulin level is unable to enter the cell through tyrosine kinase receptors present on cell membrane [19]. Most oral hypoglycaemic either stimulate the beta cells of the pancreas causing the enhanced release of insulin or increase the sensitivity of insulin receptors to overcome the insulin resistance. Over the years or decades, these pancreatic beta cells and receptor protein are exhausted and depleted, that cannot be resynthesized by medicines; thus, a diabetic individual could not revert to a normal physiological condition. These individuals will develop DR as a microvascular complication if they have a genetic susceptibility for ALDR precursor gene. Once DR is established, it will progress to NPDR after prolonged hyperglycaemic condition due to the activation and increased production of ALDR-2 enzyme [3]. NPDR progresses to PDR stage if proper treatment is not given to the patient.
The possible mechanism for progression of DR due to dyslipidemia could be the activation of PKC and AGE pathways [20]. Typically, PKC is involved in a number of physiological processes in our body, but it’s up regulation contributes to the pathogenesis of DR by: (i) differential synthesis of proteins and remodelling of Extracellular Matrix (ECM); and (ii) increasing angiogenic factors – both of which cause capillary occlusion and leukostasis due to endothelial dysfunction and altered retinal blood flow [21]. PKC family has 10 isoforms of which 1/2 isoform is strongly associated with the progression of DR [21]. De novo synthesis of Diacylglycerol (DAG) ensues as a by-product, when hyperglycaemia leads to an increase in glucose flux through the glycolysis pathway. Furthermore, the accumulated long-chain Fatty Acids (FAs) due to dyslipidemia instantaneously transforms into DAG. This disproportionate production of DAG activates PKC enzymes [22].
On the other hand, AGEs are constantly produced, non-enzymatically, from reducing sugars and lipoproteins, at a slower rate since the embryonic development that gets accumulated over time. Their production is markedly augmented in diabetes because of the sustained hyperglycaemia [23]. Carboxyethylpyrrole [24] and malondialdehyde [25] are the two types of AGEs product associated with the pathogenesis of DR. Their serum levels significantly correlate with the severity of the retinopathy. The interaction of AGEs with their specific cell surface G-Protein Coupled Receptors (GPCR) like RAGE, galectin-3, CD36, and the macrophage scavenger receptor has been linked with the advancement of DR [26]. An animal model study done on rats by Hammes HP et al., indicated that the accumulation of AGEs in the retinal capillaries and loss of pericytes cells occurred within 26 weeks of the diabetes establishment. Treatment with AGEs inhibitor significantly reduced AGEs accumulation, microaneurysms, acellular capillaries and pericyte cell loss in diabetic individuals [27].
Our results is in agreement with few previous studies [28-34] that indirectly correlated the significance of LDL-C, TC and TG in the progression of DR. ETDRS Report 22 (1996) showed that the patients who had increase serum TC or higher serum LDL-C levels were more prone to have retinal hard exudate [28]. The Atherosclerosis Risk in Communities Study (2002) concluded that the presence of retinal hard exudates was associated with the plasma LDL-C and plasma lipids [29]. The Cardiovascular Health Study (2002) demonstrated that DR was associated with higher mean Systolic Blood Pressure (SBP), plasma TC, LDL-C and Cardiovascular Disease (CVD) presence; this study further specified that elevations in plasma LDL-C might contribute to the hard exudate deposition in the retina of diabetics [30].
A North Indian observational case-control study associated NPDR with Clinically Significant Macular Oedema (CSME) and dyslipidemia. On univariate analysis, retinal hard exudates were significantly associated with SBP, serum TC, serum LDL-C, and serum TG levels. However, on linear regression analysis, serum TC and serum LDL-C was established as an independent risk factor affecting the density of retinal hard exudate [31]. A prospective study of serum lipids and risk of diabetic macular oedema in Type 1 diabetes found that TC to HDL-C ratio and LDL-C separately were linked to an increased threat of CSME and retinal hard exudate; it was concluded that treatment with lipid-lowering agent among Type 1 diabetic subjects might decline the risk of the CSME [32]. SN-DREAMS Report Number 13 revealed that high serum LDL-C, non-HDL-C, and TC was related to CSME and non-CSME [33]. Another study underlined the association of dyslipidemia with macular oedema and retinal hard exudates in Type 2 diabetes patients. A statistically significant high levels of TG, TC, total lipids and cholesterol ester was found in the diabetic patients with manifested maculopathy as compared to those without diabetic maculopathy [34].
Some treatment trial studies also signify the role of dyslipidemia in the progression of DR. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study concluded that intensive combination treatment of dyslipidemia along with intensive glycaemic control reduced the rate of DR progression [35]. Gordon B et al., reported the effects of lipid lowering on DR and concluded that reduction in TC and LDL-C might have a beneficial effect on background retinopathy in diabetics [36]. Sen K et al., reported that an HMG-CoA reductase inhibitor significantly retarded the progression of retinopathy in diabetics with hypercholesterolemia [37].
Limitation
We estimated serum lipid level and correlated it with progression of DR, which is an indirect method. Study could have been more precise if the levels of various lipid markers would have been estimated in the vitreous humor that could be collected during vitrectomy indicated for patients of diabetic retinopathy.
Conclusion
Diabetic Retinopathy will occur in all genetically susceptible diabetics eventually. The persistent hyperglycaemia stimulates diverse molecular mechanisms, including increased ALDR-2 activity that results in the establishment of DR. However, the progression of DR is largely regulated by the mechanisms that are likely to be mediated by dyslipidemia rather than hyperglycaemia alone. Based on the results of our study, it can be concluded that the lipid lowering drugs as an adjunctive to the traditional diabetes therapy that involves rigorous blood sugar and blood pressure control, might retard the progression of DR.
*significant, NDNR; No diabetes and no retinopathy, DNR; Diabetic but no retinopathy, NPDR; Non-proliferative diabetic retinopathy, PDR; Proliferative diabetic retinopathy, FBS; Fasting blood sugar, PPS; Post prandial sugar, TG; Triglyceride, TC; Total cholesterol, HDL-C; High density lipoprotein cholesterol, LDL-C; Low density lipoprotein cholesterol, VLDL; Very low density lipoprotein, ALDR; Aldose reductase.
[1]. Hodgkinson AD, Søndergaard KL, Yang B, Cross DF, Millward BA, Demaine AG, Aldose reductase expression is induced by hyperglycaemia in diabetic nephropathyKidney Int 2001 60(1):211-18.10.1046/j.1523-1755.2001.00788.x11422753 [Google Scholar] [CrossRef] [PubMed]
[2]. Gupta P, Verma N, Bhattacharya S, Mahdi AA, Usman K, Tiwari S, Association of diabetic autonomic neuropathy with red blood cell aldose reductase activityCan J Diabetes 2014 38(1):22-25.10.1016/j.jcjd.2013.07.02124485209 [Google Scholar] [CrossRef] [PubMed]
[3]. Reddy GB, Satyanarayana A, Balakrishna N, Ayyagari R, Padma M, Viswanath K, Erythrocyte aldose reductase activity and sorbitol levels in diabetic retinopathyMol Vis 2008 14:593-601. [Google Scholar]
[4]. Antonetti DA, Klein R, Gardner TW, Diabetic retinopathyN Engl J Med 2012 366(13):1227-39.10.1056/NEJMra100507322455417 [Google Scholar] [CrossRef] [PubMed]
[5]. Adamiec-Mroczek J, Oficjalska-Młyńczak J, Misiuk-Hojło M, Proliferative diabetic retinopathy-the influence of diabetes control on the activation of the intraocular molecule systemDiabetes Res Clin Pract 2009 84(1):46-50.10.1016/j.diabres.2009.01.01219237221 [Google Scholar] [CrossRef] [PubMed]
[6]. Kempen JH, O’Colmain BJ, Leske MC, Haffner SM, Klein R, Moss SE, The prevalence of diabetic retinopathy among adults in the United StatesArch Ophthalmol 2004 122(4):552-63.10.1001/archopht.122.4.55215078674 [Google Scholar] [CrossRef] [PubMed]
[7]. Jenchitr W, Samaiporn S, Lertmeemongkolchai P, Chongwiriyanurak T, Anujaree P, Chayaboon D, Prevalence of diabetic retinopathy in relation to duration of diabetes mellitus in community hospitals of LampangJ Med Assoc Thai 2004 87(11):1321-26. [Google Scholar]
[8]. Mara Lorenzi, The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilientExp Diabetes Res 2007 2007:6103810.1155/2007/6103818224243 [Google Scholar] [CrossRef] [PubMed]
[9]. Mathebula SD, Polyol pathway: A possible mechanism of diabetes complications in the eyeAfrican Vision and Eye Health 2014 74(1):1-5.10.4102/aveh.v74i1.13 [Google Scholar] [CrossRef]
[10]. Noh H, King GL, The role of protein kinase C activation in diabetic nephropathyKidney Int Suppl 2007 (106):S49-53.10.1038/sj.ki.500238617653211 [Google Scholar] [CrossRef] [PubMed]
[11]. Giacco F, Brownlee M, Oxidative stress and diabetic complicationsCirc Res. 2010 107(9):1058-70.10.1161/CIRCRESAHA.110.22354521030723 [Google Scholar] [CrossRef] [PubMed]
[12]. Kador PF, Randazzo J, Blessing K, Makita J, Zhang P, Yu K, Polyol formation in cell lines of rat retinal capillary pericytes and endothelial cells (TR-rPCT and TR-iBRB)J Ocul Pharmacol Ther 2009 25(4):299-307.10.1089/jop.2008.007019450153 [Google Scholar] [CrossRef] [PubMed]
[13]. Vistoli G, De Maddis D, Cipak A, Zarkovic N, Carini M, Aldini G, Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formationFree Radic Res 2013 47(Suppl 1):3-27.10.3109/10715762.2013.81534823767955 [Google Scholar] [CrossRef] [PubMed]
[14]. Sasongko MB, Wong TY, Nguyen TT, Kawasaki R, Jenkins A, Shaw J, Serum apolipoprotein AI and B are stronger biomarkers of diabetic retinopathy than traditional lipidsDiabetes Care 2011 34(2):474-79.10.2337/dc10-079321270203 [Google Scholar] [CrossRef] [PubMed]
[15]. World Health Day 2016: Diabetes [Internet]. South-East Asia Regional Office. 2018 [cited 18 January 2018]. Available from: http://www.searo.who.int/india/mediacentre/events/2016/en/. [Google Scholar]
[16]. Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology.1991;98(5 Suppl):786-806. Available at: http://www.aaojournal.org/article/S0161-6420(13)38012-9/pdf10.1016/S0161-6420(13)38012-9 [Google Scholar] [CrossRef]
[17]. Suryanarayana P, Kumar PA, Saraswat M, Petrash JM, Reddy GB, Inhibition of aldose reductase by tannoid principles of Emblica officinalis: implications for the prevention of sugar cataractMol Vis 2004 10:148-54. [Google Scholar]
[18]. Kao YL, Donaghue K, Chan A, Knight J, Silink M, A novel polymorphism in the aldose reductase gene promoter region is strongly associated with diabetic retinopathy in adolescents with Type 1 diabetesDiabetes 1999 48(6):1338-40.10.2337/diabetes.48.6.133810342825 [Google Scholar] [CrossRef] [PubMed]
[19]. Lee J, Pilch PF, The insulin receptor: structure, function, and signalingAm J Physiol 1994 266(2 Pt 1):C319-34.10.1152/ajpcell.1994.266.2.C3198141246 [Google Scholar] [CrossRef] [PubMed]
[20]. Safi SZ, Qvist R, Kumar S, Batumalaie K, Ismail IS, Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targetsBiomed Res Int 2014 2014:80126910.1155/2014/80126925105142 [Google Scholar] [CrossRef] [PubMed]
[21]. Geraldes P, King GL, Activation of protein kinase C isoforms and its impact on diabetic complicationsCirc Res 2010 106(8):1319-31.10.1161/CIRCRESAHA.110.21711720431074 [Google Scholar] [CrossRef] [PubMed]
[22]. Wang QJ, PKD at the cross roads of DAG and PKC signalingTrends Pharmacol Sci 2006 27(6):317-23.10.1016/j.tips.2006.04.00316678913 [Google Scholar] [CrossRef] [PubMed]
[23]. Stitt AW, AGEs and diabetic retinopathyInvest Ophthalmol Vis Sci 2010 51(10):4867-74.10.1167/iovs.10-588120876889 [Google Scholar] [CrossRef] [PubMed]
[24]. Fathallah L, Obrosova IG, Increased retinal lipid peroxidation in early diabetes is not associated with ascorbate depletion or changes in ascorbate redox state.Exp Eye Res 2001 72(6):719-23.10.1006/exer.2001.099411384160 [Google Scholar] [CrossRef] [PubMed]
[25]. Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for agerelated macular degenerationJ Biol Chem 2003 278(43):42027-35.10.1074/jbc.M30546020012923198 [Google Scholar] [CrossRef] [PubMed]
[26]. Zong H, Ward M, Stitt AW, AGEs, RAGE, and diabetic retinopathyCurr Diab Rep 2011 11(4):244-52.10.1007/s11892-011-0198-721590515 [Google Scholar] [CrossRef] [PubMed]
[27]. Hammes HP, Martin S, Federlin K, Geisen K, Brownlee M, Amino guanidine treatment inhibits the development of experimental diabetic retinopathyProc Natl Acad Sci USA 1991 88(24):11555-58.10.1073/pnas.88.24.115551763069 [Google Scholar] [CrossRef] [PubMed]
[28]. Chew EY, Klein ML, Ferris FL, Remaley NA, Murphy RP, Chantry K, Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22.Arch Ophthalmol. 1996 114(9):1079-84.10.1001/archopht.1996.011001402810048790092 [Google Scholar] [CrossRef] [PubMed]
[29]. Klein R, Sharrett AR, Klein BE, Moss SE, Folsom AR, Wong TY, The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: the atherosclerosis risk in communities studyOphthalmology 2002 109(7):1225-34.10.1016/S0161-6420(02)01074-6 [Google Scholar] [CrossRef]
[30]. Klein R, Marino EK, Kuller LH, Polak JF, Tracy RP, Gottdiener JS, The relation of atherosclerotic cardiovascular disease to retinopathy in people with diabetes in the Cardiovascular Health StudyBr J Ophthalmol 2002 86(1):84-90.10.1136/bjo.86.1.8411801510 [Google Scholar] [CrossRef] [PubMed]
[31]. Sachdev N, Sahni A, Association of systemic risk factors with the severity of retinal hard exudates in a north Indian population with type 2 diabetesJ Postgrad Med 2010 56(1):3-6.10.4103/0022-3859.6241920393241 [Google Scholar] [CrossRef] [PubMed]
[32]. Miljanovic B, Glynn RJ, Nathan DM, Manson JE, Schaumberg A, A prospective study of serum lipids and risk of diabetic macular oedema in type 1 diabetes.Diabetes 2004 53(11):2883-92.10.2337/diabetes.53.11.288315504969 [Google Scholar] [CrossRef] [PubMed]
[33]. Raman R, Rani PK, Kulothungan V, Rachepalle SR, Kumaramanickavel G, Sharma T, Influence of serum lipids on clinically significant versus nonclinically significant macular oedema: SN-DREAMS Report number 13Ophthalmology 2010 117(4):766-72.10.1016/j.ophtha.2009.09.00520045565 [Google Scholar] [CrossRef] [PubMed]
[34]. Golubovic-Arsovska M, Association of dyslipidaemia with macular oedema and hard exudates in diabetic maculopathy.Prilozi 2007 28(2):149-60. [Google Scholar]
[35]. ACCORD Study Group. ACCORD Eye Study GroupChew EY, Ambrosius WT, Davis MD, Danis RP, Effects of medical therapies on retinopathy progression in type 2 diabetesN Engl J Med 2010 363:233-44.10.1056/NEJMoa100128820587587 [Google Scholar] [CrossRef] [PubMed]
[36]. Gordon B, Chang S, Kavanagh M, Berrocal M, Yannuzzi L, Robertson C, The effect of lipid lowering on diabetic retinopathyAm J Ophthalmo 1991 112(4):385-91.10.1016/S0002-9394(14)76244-0 [Google Scholar] [CrossRef]
[37]. Sen K, Misra A, Kumar A, Pandey RM, Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemiaDiabetes Res Clin Pract 2002 56(1):1-11.10.1016/S0168-8227(01)00341-2 [Google Scholar] [CrossRef]