Cerebral Microbleeds with Posterior Reversible Encephalopathy Syndrome: A Rare complication in Acute Lymphoblastic Leukaemia
Aravind Yogabalan1, Karthick Udupa2
1 Senior Resident, Department of General Medicine, Saveetha Medical College and Hospital, Kancheepuram, Tamil Nadu, India.
2 Associate Professor, Department of Medical Oncology, Kasturba Medical College and Hospital, Manipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Aravind Yogabalan, 140, Appinaickanpatti, Uthangarai, Krishnagiri-635207, Tamil Nadu, India.
E-mail: aravindyin@gmail.com
Cerebral microbleeds are a rare entity usually seen in hypertensive microangiopathy or amyloid angiopathy. Its occurrence in Acute Lymphoblastic Leukaemia (ALL) has not been reported so far. Clinicians while treating patients should be aware of such complications for timely diagnosis and management. This is a case report on 19-year-old male, who was on treatment for ALL, under induction phase of the Berlin-Frankfurt-Munster-95 (BFM-95) regimen, who developed one episode of generalised tonic-clonic seizures. The MRI brain in T2 sequence showed hyperintense lesions in the white matter of occipital region, suggestive of Posterior Reversible Encephalopathy Syndrome (PRES). Susceptibility Weighted Imaging (SWI) showed multifocal areas of blooming in bilateral cerebral hemispheres suggestive of microbleeds. The fibrinogen level was very low strongly suggestive of L-Asparaginase induced microbleeds. The patient was treated with antiepileptic and cryoprecipitate transfusion to maintain fibrinogen levels and further doses of L-Asparaginase were withheld. He recovered without any neurological sequelae.
L-Asparaginase, Microangiopathy, Tonic-clonic seizures
Case Report
A 19-year-old male was diagnosed with ALL. He had no significant medical history. He was initiated treatment for ALL under standard BFM-95 regimen. In the induction phase, he was treated with vincristine, daunorubicin, prednisolone, L-Asparaginase and intrathecal methotrexate. His baseline renal function, liver function and coagulation profile were normal. Complete haemogram showed haemoglobin of 7.8 gm/dL, total WBCs of 4.3 lakhs/dL and platelets of 37,000/dL. Lumbar puncture showed acellular smear. He was monitored regularly for toxicity of the chemotherapeutic drugs including complete blood counts, coagulation profile Prothrombin Time (PT), Activated Partial Thromboplastin Time (APTT) and fibrinogen assays, liver and renal function tests. Packed cell transfusions were given when his haemoglobin dropped below 7 gm/dL. His lowest platelet count during the treatment was 18,000/dL but he had no bleeding manifestations and his platelet counts improved spontaneously. As per the protocol, nine doses of L-Asparaginase has to be given. Before the 6th dose of L-Asparaginase, his fibrinogen assay was found to be <50 mg/dL. Platelet count was 88,000/dL and PT, APTT were normal. So correction of fibrinogen level with target level of 150 mg/dL was achieved by transfusion of cryoprecipitate and L-Asparaginase was administered.
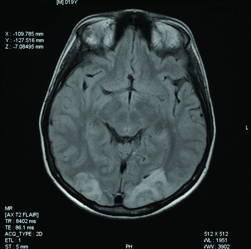
Two days after the administration of L-Asparaginase, he developed generalised tonic-clonic seizures lasting for about one minute which subsided following administration of lorazepam. His blood pressure was normal. There were no residual neurological deficits but patient had complaints of blurring of vision. To rule out other metabolic causes of seizures, serum levels of calcium, magnesium and sodium were done, which were all normal. The CT brain was normal. Ophthalmological evaluation including perimetry and colour vision were tested and were found to be normal. In meantime his visual symptoms were improved. Initially it was thought to be due to cortical venous thrombosis. The MR venogram was done which was also normal. The MRI brain T2 sequence showed hyper intense lesion in the white matter of the occipital region, suggestive of PRES shown in [Table/Fig-1]. The SWI sequences showed multiple areas of blooming less than 1 mm, involving both the cerebral hemispheres and cerebellum suggestive of microbleeds shown in [Table/Fig-2]. These microbleeds were not seen in other sequences.
The MRI brain T2 sequence showing hyper intense lesion in the white matter of the occipital region.
Susceptibility weighted imaging sequence showing multiple areas of microbleeds involving the cerebral hemispheres and cerebellum.
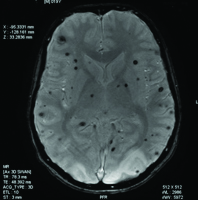
This was considered probably L-Asparaginase induced microbleeds because major bleeding is a well-known complication of L-Asparaginase and hence further doses of L-Asparaginase were not given. The patient was then continued with anti-epileptic drugs and other symptomatic treatment. He recovered without any neurological deficits. Post one year of this episode patient was in eight monthly maintenance of BFM-95 protocol, and his disease was in remission. Repeat MRI brain was normal.
Discussion
L-Asparaginase is used in the induction phase of BFM-95 regimen of ALL. Bleeding and thrombosis are well known complications. It is due to deficiency of plasma haemostatic proteins including fibrinogen, anti-thrombin and plasminogen. It can occur any time during the course or after the completion of chemotherapy but characteristically occur during third or fourth week of chemotherapy [1,2]. Only major haemorrhage and cortical venous sinus thrombosis have been reported so far [3]. Microbleeds caused by L-Asparaginase are not well known. These microbleeds are usually best seen in SWI sequences as multiple areas of punctuate blooming.
The differential diagnoses of these microbleeds are chronic hypertensive encephalopathy (hypertensive micro angiopathy), cerebral amyloid angiopathy and multiple cavernous malformations. The distribution of these microbleeds in each of these conditions varies. Importance of recognising these microbleeds lies in the fact that it is a strong predictor of occurrence of major haemorrhage if left untreated [4]. The present patient was a non-hypertensive with previous normal blood pressure recordings. The clinical picture fit into a diagnosis of coagulopathy induced microbleeds, as the patient had very low fibrinogen and was on a chemotherapeutic drug L-Asparaginase, which was well known to cause bleeds.
PRES and L-Asparaginase
PRES is a clinico-radiological entity characterised usually by reversible vasogenic oedema involving mainly the white matter of the occipital and parietal regions. The global incidence of PRES is not known. It is found to occur in all age groups. The pathogenesis of PRES is thought to be due to breakdown in blood brain barrier resulting in vasogenic oedema. The causes include accelerated hypertension, eclampsia, chemotherapeutic drugs, immunosuppressants and vasculitis [5]. L-Asparaginase induced PRES is reported only in few case reports and series. It is more common in the paediatric age group [6]. In present case, microbleeds seen on MRI throughout the brain possibly suggest a disruption of the blood brain barrier through which the chemotherapeutic drug would have been caused the vasogenic oedema especially of the occipital and parietal regions and caused the so called posterior reversible encephalopathy syndrome. Case reports of L-Asparaginase induced fatal toxic leukoencephalopathy also has been reported [7].
For diagnosis and follow up of major haemorrhage or thrombosis both CT and MRI scan of the brain can be used [8]. The MRI brain with SWI sequence can be helpful in case of microbleeds that cannot be seen by CT scan and other sequences of MRI Brain. Most patients recover without any major neurological sequela. Treatment of PRES include anti-epileptics and other symptomatic measures. For major haemorrhages and microbleeds, transfusion of cryoprecipitate to maintain the levels of fibrinogen above 150 mg/dL is recommended. It is better to withhold further doses of L-Asparaginase.
Conclusion
L-Asparaginase induced PRES and microbleeds are rare entity. Monitoring of fibrinogen assay in addition to routine tests, for patients on L-Asparaginase could recognise and prevent any serious Central Nervous System (CNS) complications and neurological deficits.
[1]. Feinberg WM, Swenson MR, Cerebrovascular complications of L-asparaginase therapyNeurology 1988 38(1):127-33.10.1212/WNL.38.1.1273275903 [Google Scholar] [CrossRef] [PubMed]
[2]. Priest JR, Ramsay NK, Steinherz PG, Tubergen DG, Cairo MS, Sitarz AL, A syndrome of thrombosis and hemorrhage complicating L-asparaginase therapy for childhood acute lymphoblastic leukemiaJ Pediatr 1982 100(6):984-89.10.1016/S0022-3476(82)80535-0 [Google Scholar] [CrossRef]
[3]. Wani NA, Kosar T, Pala NA, Qureshi UA, Sagittal sinus thrombosis due to L-asparaginaseJ Pediatr Neurosci 2010 5(1):32-35.10.4103/1817-1745.6668321042505 [Google Scholar] [CrossRef] [PubMed]
[4]. Tsushimaa Y, Aokib J, Endob K, Brain Micro hemorrhages Detected on T2*- Weighted Gradient-Echo MR ImagesAm J Neuroradiol 2003 24:88-96. [Google Scholar]
[5]. Legriel S, Pico F, Azoulay E, Understanding posterior reversible syndrome, Annual update in intensive care and emergency medicine 2011 1:631-53.10.1007/978-3-642-18081-1_56 [Google Scholar] [CrossRef]
[6]. Appachu MS, Purohit S, Lakshmaiah KC, Kumari BSA, Appaji L, Posterior reversible encephalopathy syndrome in pediatric acute leukemia: Case series and literature reviewIndian J Med Paediatr Oncol 2014 35(1):79-82.10.4103/0971-5851.13372725006290 [Google Scholar] [CrossRef] [PubMed]
[7]. Frantzeskaki F, Rizos M, Papathanassion N, Lerikou M, Armaganidis A, L-asparaginase fatal toxic encephalopathy during consolidation treatment in an adult with acute lymphoblastic leukemiaAm J Case Rep 2013 14:311-14.10.12659/AJCR.88926823961306 [Google Scholar] [CrossRef] [PubMed]
[8]. Fleischhack G, Solymosi L, Reiter A, CBender-Gğtze C, Eberl W, Bod U, Imaging methods in diagnosis of cerebrovascular complications with L-asparaginase therapyKlin Padiatr 1994 206(4):334-41.10.1055/s-2008-10466267967435 [Google Scholar] [CrossRef] [PubMed]