Introduction
β-Thalassaemia (βTM) is the most common chronic haemolytic anaemia in Egypt [1]; as a consequence of reduced or absent synthesis of beta globin chains, there is an in imbalance of globin chains causing haemolysis and impaired erythropoiesis leading to affected children to acquire lifelong blood transfusions [2]. However, multiple blood transfusions can lead to iron overload resulting in endocrine dysfunction that might disturb single or multiple endocrine glands [3].
Management strategies have improved the survival and complication-free survival in βTM. However, many transfusion-dependent patients continue to develop iron-induced cardiomyopathy which is responsible for tissue damage and finally death [4]. Myocardial siderosis was observed in nearly one-third of βTM patients [5]. Iron toxicity may indirectly affect heart function by damaging other organs in varying degree as thyroid gland causing hypothyroidism [6]. Hypothyroidism can lead to bradycardia, decreased LV function, pericardial effusion, and increased peripheral vascular resistance [7].
Thyroid dysfunction is frequently present in βTM, but its prevalence and severity varies in different cohorts, and its long-term natural history is poorly understood [8]. Hypothyroidism includes primary hypothyroidism, subclinical hypothyroidism, as well as secondary (central) hypothyroidism.
In a study of Gharzuddine WS and his coworkers, for LV function in βTM patients, LV by Echocardiogram (echo), reported variable abnormalities depending on the patient’s age and disease severity [9]. Early detection of cardiac-function impairment can help in preventing further cardiac damage by modifying disease progression and treatment.
We aimed to assess cardiac function in transfusion dependent βTM children in Qena, Egypt, using conventional echocardiogram and correlate findings with thyroid hormones {free thyroxine (FT4) and Thyroid Stimulating Hormone (TSH)} and serum ferritin levels.
Materials and Methods
This was a cross-sectional study conducted between January 2016 and December 2016. The study involved 40 transfusion-dependent βTM cases aged from 6-12 years who were on regular follow up in the outpatient clinic. Sample size was calculated at power 80%, to be at least 36. In addition, apparently 15 healthy age and sex-matched children with no hepatic or renal disease, had normal echocardiogram, and negative blood tests for thalassaemia were considered for the control group. They were included from the outpatient clinic of Paediatric Department, Qena University Hospital. A written informed consent was obtained from the parents of each child. The study was approved by the ethics and Research committee of Qena faculty of medicine and in accordance with Declaration of Helsinki.
Children diagnosed with βTM major, were included in the study aged from 1 to 15 years. Diagnosis of βTM was based on clinical presentation, family history, haematological indices and haemoglobin electrophoresis.
All of the included cases were subjected to full medical history evaluation and complete clinical examination, including age, sex, weight, height, disease duration, first time of blood transfusion, number of blood transfusions/year, history of splenectomy, type and duration of chelation therapy. All cases were on regular blood transfusion with 10-15 mL/kg packed cells every 21-25 days, and iron chelation therapy with subcutaneous desferrioxamine (40 mg/kg/day administered through a battery-operated portable pump over a period of 8-12 hours overnight, for 5-7 nights/week).
According to mean pretransfusional haemoglobin level, βTM major cases were subdivided into adequately transfused group (with mean pretransfusional haemoglobin ≥9 g/dL) and inadequately transfused group (with mean pretransfusional haemoglobin <9 g/dL).
According to the mean serum ferritin level, βTM major cases were subdivided into well-chelated group (with mean serum ferritin <2500 ng/mL) and poorly chelated group (with mean serum ferritin ≥2500 ng/mL).
Patients with βTM minor and intermedia and those with acute illness, any hormonal therapy or severe liver disease or end-stage renal disease with creatinine clearance <30% of normal or advanced heart failure or hypertensive cardiomyopathy; subjects with a family history of thyroid dysfunction, genetic, metabolic or neurodegenerative disease or with other medical illnesses have known to impact the primary organ systems of interest were also excluded; e.g., congenital heart defects.
Both cases and controls were subjected to detailed history taking and thorough clinical examination with special emphasis on growth parameters according to the Egyptian growth curves and signs of hypothyroidism, and were subjected to:
Blood sampling: Five mL blood was drawn from each patient and control and divided into: 2 mL on Ethylene Di-Amine Tetra-Acetic acid (EDTA) tube for blood count using Cell dyne 1800 cell counter (Abbott diagnostics, USA), reticulocyte count and haemoglobin electrophoresis (using automated cation-exchange High-Performance Liquid Chromatography (HPLC) D-10 provided by Bio-Rad Laboratories (Bio-Rad Laboratories, USA). 3 mL blood put in plain tube allowed to clot and then centrifuged for 10 minutes at 3,000 rpm and serum is separated for the analysis of serum ferritin, TSH and FT4 using automated Chemiluminescent Microparticle Immune Assay (CMIA) utilizing ChemiFlex Technology (Architect i2000, Abbott diagnostics, USA). Reference range was 0.43-3.28 mU/L for TSH and for FT4 10.80-16.80 pmol/L. Diagnosis of thyroid dysfunction was based on the following criteria; Primary hypothyroidism when FT4 is <12 pmol/L and TSH is >4 mIU/mL; subclinical hypothyroidism when FT4 is normal and TSH is >4 mIU/mL; central (secondary) hypothyroidism when FT4 is <12 pmol/L and TSH is low or normal.
Conventional two-dimensional (2D) Doppler echocardiography: (Vivid S5, Germany, syncmaster 450 MB) with a 2.0-3.5 MHz transducer; utilizing pulsed Doppler, M mode and continuous Doppler on TR jet was used to assess the following echo parameters as implemented by [10]:
Dimensions of systolic function: the LVESD thyroxinand diastole (LVEDD), EF% was measured using Teichholz formula and fractional shortening (FS%).
Evaluation of diastolic function: The LV diastolic filling indices include early diastolic wave (E-wave), late diastolic wave (A-wave), E-wave to A-wave (E/A) ratio.
The myocardial performance index: the Tei index, is a combined index of systolic and Tei LV i.e., the sum of Isovolumetric Contraction Time (IVCT) and Isovolumetric Relaxation Time (IVRT) divided by the Ejection Time (ET) and was used for quantification of global LV function. Tei LV index is considered abnormal when exceeds 0.30.
Pulmonary artery pressure: using the TR jet (where pulmonary artery systolic pressure was calculated from the pressure gradient through the tricuspid valve using the Bernoulli equation and by adding the right atrial pressure to the pressure gradient of TR jet; pulmonary hypertension was considered when it exceeds 36 mmHg.
Statistical Analysis
Analysis of data was done using SPSS version 22 (IBM SPSS statistics; USA) as follows: description of quantitative variables as Mean±Standard Deviation (SD) and range compared by independent t-test. Description of qualitative variables as number and percentage and will be compared by chi-square test. Pearson’s correlation coefficient was used to explore the relationship between quantitative variables. All statistical tests were two tailed, and a p-value below 0.05 was considered statistically significant.
Results
A total of 40 βTM patients 29 were males (72.5%) and 11 females (27.5%) and 15 healthy subjects as control were enrolled in the study, male (n=13, 86.7%) and female (n=2, 13.3%). None of the patients had signs and symptoms of heart failure, arrhythmia and thyroid disease and 55% were poorly chelated, summary of clinical and laboratory data [Table/Fig-1].
Clinical and laboratory data of studied cases.
| β-TM cases Personal characteristics and clinical parameter | n (%) |
|---|
| Age | <9 years >≤9 years | 17 (42.5) 23 (57.5) |
| Sex | Male Female | 29 (72.5) 11 (27.5) |
| Consanguinity | Positive Negative | 31 (77.5) 9 (22.5) |
| Family History of Similar Condition | Positive Negative | 29 (72.5) 11 (27.5) |
| Associated other chronic haemolytic anaemia | Yes No | 1 (2.5) 39 (97.5) |
| Duration of blood transfusion therapy (years) | <6 years ≤6 years | 13 (32.5) 27 (67.5) |
| Blood transfusion Frequency | Not frequent Frequent/2weeks | 33 (82.5) 7 (17.5) |
| Iron chelating agents | Irregular Regular | 20 (50) 20 (50) |
| Splenomegaly | Positive Negative | 34 (85) 6 (15) |
| Splenectomy | Done Not done | 15 (37.5) 25 (62.5) |
| Hepatomegaly | Positive Negative | 16 (40) 24 (60) |
| Signs of heart failure | Yes No | 0 40 (100) |
| Signs of arrhythmia | Yes No | 0 40 (100) |
| Signs of hypothyroidism | Yes No | 0 40 (100) |
| HB (g/dL) | <10 >10 | 39 (97.5) 1 (2.5) |
| S. ferritin (μg/dL) | <2500 ≤2500 | 16 (40) 24 (60) |
| TSH (μIU/mL) | Normal High | 34 (85) 6 (15) |
| FT4 (pmol/L) | Normal Low | 36 (87.5) 5 (12.5) |
Hb: Haemoglobin; TSH: Thyroid simulating hormone; FT4: Free thyroxinand
Total number of cases with impaired thyroid function was (n=10, 25%); serum ferritin value was significantly higher in all the four groups of thalassaemia patients [Table/Fig-2].
Pattern of thyroid hormones in relation to serum ferritin levels in βTM cases.
| Pattern of thyroid function in β-TM cases | n (%) | Ferritin (ug/dL) |
|---|
| High TSH+Low FT4 | Primary hypothyroidism | 1 (2.5%) | 6639 |
| High TSH+Normal FT4 | Subclinical hypothyroidism | 5 (12.5%) | 4065.6±2745 |
| Normal TSH+Low FT4 | Central hypothyroidism | 4 (10%) | 2609.9±2364 |
| Normal TSH+Normal FT4 | Euthyroid | 30 (75%) | 2242.7±1414 |
TSH: Thyroid simulating hormone; FT4: Free thyroxin
βTM cases had significantly higher heart rate (p-value=0.044) and ferritin level (p-value=<0.001), significant lower systolic BP (p-value=0.007), diastolic BP (p-value=0.028), Hb level (p-value=<0.001), significant larger dimensions of LVEDD (p-value=0.025), LVESD (p-value=0.046) and E-wave value (p-value=0.004) [Table/Fig-3].
Demographic data, clinical, laboratory data and echo parameters in βTM patients and controls.
| Parameter (Mean±SD) | Groups | t-test |
|---|
| βTM cases | Control | t | p-value |
|---|
| Age (years) | Mean±SD | 9.51±3.26 | 8.73±2.55 | 0.833 | 0.409 |
| Weight (kg) | Mean±SD | 23.20±6.29 | 24.07±8.71 | 0.534 | 0.596 |
| Height (cm) | Mean±SD | 120.10±14.08 | 123.47±15.40 | 0.602 | 0.549 |
| HC (cm) | Mean±SD | 51.56±1.92 | 51.27±2.49 | 0.469 | 0.641 |
| Heart rate (bpm/minute) | Mean±SD Range | 100.57±9.77 | 94.9±6.45 | 2.066 | 0.044* |
| 80-124 | 85-110 |
| Systolic BP (mmHg) | Mean±SD Range | 95.50±9.86 | 104.00±10.56 | 2.794 | 0.007* |
| 80.0-120.0 | 90.0-120.0 |
| Diastolic BP(mmHg) | Mean±SD Range | 66.25±8.07 | 71.33±5.16 | 2.266 | 0.028* |
| 50.0-80.0 | 60.0-80.0 |
| TSH (mIU/mL) | Mean±SD Range | 2.89±0.89 | 2.79±1.34 | 0.279 | 0.781 |
| 1.0-7.5 | 1.4 - 4.5 |
| Free T4 (pmol/L) | Mean±SD Range | 17.28±4.03 | 16.17±3.19 | 1.104 | 0.275 |
| 7.08-23.04 | 12.0-23.17 |
| Hb(g/dL) | Mean±SD Range | 7.65±0.90 | 12.27±0.65 | 18.106 | <0.001** |
| 5.0-11.1 | 11.0-13.0 |
| S. ferritin (ug/dL) | Mean±SD Range | 2617.2±1865.9 | 118.3±42.5 | 5.156 | <0.001** |
| 309.0-7505.0 | 65.0-210.0 |
| Dimensions of systolic function) | LVEDD (mm/M2) | 40.63±6.17 | 36.53±4.82 | 2.313 | 0.025* |
| LVESD (mm/M2) | 25.40±4.83 | 22.60±3.54 | 2.044 | 0.046* |
| LVEF % | 67.68±7.04 | 67.93±7.17 | -0.121 | 0.904 |
| FS% | 38.05±5.70 | 37.53±5.88 | 0.297 | 0.768 |
| Dimensions of diastolic function | E wave (cm/sec) | 114.78±20.16 | 97.67±14.67 | 2.995 | 0.004* |
| A wave (cm/sec) | 57.05±18.91 | 53.20±13.85 | 0.718 | 0.476 |
| E/A ratio | 2.20±0.69 | 1.91±0.43 | 1.538 | 0.130 |
| (Tei LV) | 0.29±0.32 | 0.34±0.14 | -0.555 | 0.581 |
| Pulmonary artery systolic pressure | 37.79±9.21 | 32.80±6.34 | 1.928 | 0.059 |
*significant; Head circumference: HC TSH: Thyroid simulating hormone; FT4: Free thyroxin; LVEDD: Left ventricle end diastolic diameter; LVESD: Left ventricle end systolic diameter; LVEF: Left ventricular ejection fraction; FS: Fractional shortening; E wave: Early diastolic flow velocity; A wave: Late diastolic flow velocity
Compared to controls; all βTM cases had significant higher mitral and filling velocity E-wave velocity (p-value<0.05) and βTM cases with impaired thyroid function had significant larger dimensions of LVEDD and LVESD, significant higher LV Tei, significant higher pulmonary artery pressure and significant lower EF % [Table/Fig-4].
Thyroid function status in the studied cases in relation to laboratory and echocardiographic parameters.
| Parameter (Mean ± SD) | Thyroid function in cases | Control | p-value |
|---|
| Impaired | Euthyroid |
|---|
| Age (years) | 10.5±3.14 | 9.18±3.29 | 8.82±2.68 | 0.1349a 0.6451b 0.2747c |
| Transfusion therapy duration (years) | 9.35±3.06 | 7.53±3.55 | - | 0.1558 |
| Hb (g/dL) | 7.88±0.42 | 7.57±1.01 | 12.14±0.67 | <0.00001a <0.00001b 0.3481c |
| Ferritin (ug/dL) | 4217±2375 | 2243±1414 | 121.36± 47.49 | <0.00001a* <0.00001b* 0.0028c* |
| Dimensions of systolic function | LVEDD (mm/M2) | 42.1±6.79 | 40.13±5.99 | 36.36±5.18 | 0.0247a* 0.0496b* 0.3894c |
| LVESD (mm/M2) | 27.7±5.58 | 24.63±4.39 | 21.91±3.11 | 0.0100a* 0.1271b 0.0819c |
| LVEF % | 63.9±6.30 | 68.93±6.91 | 69.36±7.30 | 0.1621a 0.6534b 0.0487c* |
| FS% | 35.2±4.96 | 39.0±5.68 | 38.64±6.20 | 0.3128a 0.4240b 0.0670c |
| Dimensions of diastolic function | E wave (cm/second) | 114.3±17.37 | 115.93±18.78 | 99.64±12.56 | 0.0167a* 0.002b* 0.8098c |
| A wave (cm/second) | 61.2±26.05 | 55.57±16.31 | 55.73±15.43 | 0.3266a 0.4236b 0.4236c |
| E/A ratio | 2.148±0.90 | 2.22±0.62 | 1.87±0.41 | 0.3761a 0.0888b 0.7894c |
| (Tei LV) | 0.48±0.55 | 0.24±0.14 | 0.20±0.05 | 0.0392a* 0.5241b 0.0327c* |
| Pulmonary artery systolic pressure | 40.5±10.47 | 36.97±8.60 | 31.91±6.25 | 0.03097a* 0.1041b 0.2932c |
*significant; a: impaired vs. control; b: normal function vs. control; c: impaired vs. normal function)
TSH: Thyroid simulating hormone; FT4: Free thyroxin; LVEDD: Left ventricle end diastolic diameter; LVESD: Left ventricle end systolic diameter; LVEF: Left ventricular ejection fraction; FS: Fractional shortening; E wave: Early diastolic flow velocity; A wave: Late diastolic flow velocity
With increasing age; β-TM cases aged above nine years showed significant increase in LVEDD, β-TM cases with impaired thyroid function exhibited significantly higher LVESD in comparison to normal (p-value=0.001), and serum ferritin (p-value=0.01) [Table/Fig-5].
Thyroid hormones, ferritin and echocardiographic parameters in the studied βTM cases in relation to age.
| Parameter (Mean±SD) | βTM Cases Age | t-test |
|---|
| <9 years | >9 years | t | p-value |
|---|
| TSH | (uIU/mL) | 2.77±1.48 | 2.80±1.25 | 0.076 | 0.940 |
| FT4 | (p mol/L) | 16.99±4.25 | 17.89±3.73 | 0.814 | 0.421 |
| Ferritin | (ug/dl) | 1765.6±1202.0 | 3246.6±2036.3 | 2.6694 | 0.011* |
| Dimensions of systolic function | LVEDD (mm/M2) | 36.65±3.32 | 43.57±6.18 | -4.184 | 0.001** |
| LVESD (mm/M2) | 22.65±2.45 | 27.43±5.18 | -3.524 | 0.001** |
| LVEF % | 68.47±5.95 | 67.09±7.82 | 0.610 | 0.546 |
| FS% | 39.00±4.62 | 37.35±6.39 | 0.905 | 0.371 |
| Dimensions of diastolic function | E wave (cm/sec) | 116.88±16.90 | 113.22±22.51 | 0.563 | 0.576 |
| A wave (cm/sec) | 56.71±18.05 | 57.30±19.91 | -0.098 | 0.923 |
| E/A ratio | 2.25±0.72 | 2.16±0.67 | 0.429 | 0.670 |
| Tei LV | 0.31±0.46 | 0.27±0.12 | 0.415 | 0.681 |
| Pulmonary artery systolic pressure | 39.59±8.56 | 36.41±9.64 | 1.072 | 0.291 |
*significant; TSH- Thyroid simulating hormone; FT4- Free thyroxin; LVEDD- Left ventricle end diastolic diameter; LVESD- Left ventricle end systolic diameter; LVEF- Left ventricular ejection fraction; FS- Fractional shortening; E- Early diastolic flow velocity; A- Late diastolic flow velocity
The group with a duration of blood transfusion therapy more than six years; showed significantly larger dimensions of LVEDD (p-value=0.002), LVESD (p-value=0.018) [Table/Fig-6].
Thyroid function, serum ferritin and echo parameters in βTM patients in relation to duration of blood transfusion therapy.
| Parameter (Mean±SD) | βTM cases duration of blood transfusion therapy (years) | t-test |
|---|
| <6 years | >6 years | t | p-value |
|---|
| TSH | (uIU/mL) | 2.74±1.19 | 2.88±1.65 | 0.323 | 0.749 |
| FT4 | (p mol/L) | 17.50±2.45 | 17.50±4.50 | 0.042 | 0.967 |
| Ferritin | (ug/dl) | 2031.6±1277.5 | 2942.7±2024.1 | -1.482 | 0.1467 |
| Dimensions of systolic function | LVEDD (mm/M2) | 36.38±3.38 | 42.67±6.20 | 3.402 | 0.002* |
| LVESD (mm/M2) | 22.85±2.85 | 26.63±5.14 | 2.467 | 0.018* |
| LVEF % | 67.62±6.41 | 67.70±7.44 | 0.037 | 0.971 |
| FS% | 38.08±4.92 | 38.04±6.12 | 0.020 | 0.984 |
| Dimensions of diastolic function | E wave (cm/sec) | 115.00±20.72 | 114.67±20.28 | 0.048 | 0.962 |
| A wave (cm/sec) | 55.69±18.38 | 57.70±19.46 | 0.311 | 0.757 |
| E/A ratio | 2.25±0.75 | 2.17±0.67 | 0.334 | 0.740 |
| Tei LV | 0.23±0.15 | 0.32±0.37 | 0.911 | 0.369 |
| Pulmonary artery systolic pressure | 38.54±8.78 | 37.42±9.56 | 0.353 | 0.726 |
*significant; TSH- Thyroid simulating hormone; FT4, Free thyroxin; LVEDD- Left ventricle end diastolic diameter; LVESD- Left ventricle end systolic diameter; LVEF- Left ventricular ejection fraction; FS- Fractional shortening; E- Early diastolic flow velocity; A- Late diastolic flow velocity
βTM cases with frequent blood transfusion every two weeks have significant increase in serum ferritin levels compared to non frequent blood transfusion cases [Table/Fig-7].
Thyroid hormones, serum ferritin and echo parameters in βTM patients in relation to blood transfusion frequency.
| Parameter (Mean±SD) | Blood transfusion Frequency | t-test |
|---|
| Not frequent | Frequent | t | p-value |
|---|
| Age | (years) | 10.17±3.1 | 8.42±3.69 | -1.19 | 0.2439 |
| TSH | (uIU/mL) | 2.75±0.88 | 2.79±1.42 | 0.068 | 0.946 |
| FT4 | (p mol/L) | 16.99±4.25 | 17.50±3.99 | 0.315 | 0.754 |
| Ferritin | (ug/dL) | 2456±1731 | 4203.5±1887 | 2.165 | 0.039* |
| Dimensions of systolic function | LVEDD (mm/M2) | 40.21±6.13 | 42.57±6.45 | 0.917 | 0.365 |
| LVESD (mm/M2) | 25.27±4.82 | 26.00±5.23 | 0.358 | 0.722 |
| LVEF % | 67.24±6.91 | 69.71±7.83 | 0.841 | 0.406 |
| FS% | 37.79±5.66 | 39.29±6.18 | 0.627 | 0.535 |
| Dimensions of diastolic function | E wave (cm/sec) | 112.42±21.19 | 125.86±8.53 | 1.635 | 0.110 |
| A wave (cm/sec) | 55.61±19.69 | 63.86±13.78 | 1.050 | 0.300 |
| E/A ratio | 2.23±0.72 | 2.06±0.56 | 0.585 | 0.562 |
| Tei LV | 0.31±0.34 | 0.17±0.08 | 1.038 | 0.306 |
| Pulmonary artery systolic pressure | 37.47±9.72 | 39.29±6.75 | 0.468 | 0.642 |
TSH-Thyroid simulating hormone; FT4-Free thyroxin; LVEDD-Left ventricle end diastolic diameter; LVESD-Left ventricle end systolic diameter; LVEF- Left ventricular ejection fraction; FS- Fractional shortening; E- Early diastolic flow velocity; A- Late diastolic flow velocity
The regular use of iron chelating agent in βTM patients was more frequent in younger age patients but without significant effects on serum ferritin and Echo parameters, thyroid function and serum ferritin [Table/Fig-8].
Thyroid hormones, serum ferritin and echo parameters in βTM patients in relation to iron chelating agents.
| Parameter (Mean±SD) | Iron chelating agents | t-test |
|---|
| Irregular | Regular | t | p-value |
|---|
| Age | (years) | 11.27±2.52 | 8.25±3.27 | 2.792 | 0.01* |
| TSH | (uIU/mL) | 2.97±1.37 | 2.6±1.3 | 0.864 | 0.393 |
| FT4 | (p mol/L) | 16.47±3.73 | 18.40±4.12 | 1.441 | 0.158 |
| Ferritin | (ug/dL) | 3426±2246 | 2166±1114 | 1.892 | 0.069 |
| Dimensions of systolic function | LVEDD (mm/M2) | 42.15±6.07 | 39.10±6.03 | 1.595 | 0.119 |
| LVESD (mm/M2) | 26.00±5.05 | 24.80±4.65 | 0.782 | 0.439 |
| LVEF % | 68.80±7.53 | 66.55±6.50 | 1.012 | 0.318 |
| FS% | 38.50±6.08 | 37.60±5.40 | 0.495 | 0.624 |
| Dimensions of diastolic function | E wave (cm/second) | 118.00±19.31 | 111.55±20.96 | 1.012 | 0.318 |
| A wave (cm/second) | 58.05±21.80 | 56.05±16.01 | 0.331 | 0.743 |
| E/A ratio | 2.25±0.75 | 2.15±0.63 | 0.436 | 0.666 |
| Tei LV | 0.24±0.15 | 0.34±0.41 | 1.001 | 0.324 |
| Pulmonary artery systolic pressure | 39.95±10.49 | 35.75±7.50 | 1.443 | 0.157 |
TSH-Thyroid simulating hormone; FT4-Free thyroxin; LVEDD-Left ventricle end diastolic diameter; LVESD- Left ventricle end systolic diameter; LVEF- Left ventricular ejection fraction; FS- Fractional shortening; E- Early diastolic flow velocity; A- Late diastolic flow velocity
Splenectomized patients had significant older age and higher FT4 levels [Table/Fig-9].
Thyroid hormones, serum ferritin and echo parameters in βTM patients in relation to splenectomy.
| Parameter (Mean±SD) | Splenectomy | t-test |
|---|
| No | Yes | t | p-value |
|---|
| Age | (years) | 8.79±3.1 | 11.73± 2.15 | -3.121 | 0.004* |
| TSH | (uIU/ml) | 2.43±0.99 | 3.03±1.34 | 1.488 | 0.146 |
| FT4 | (p mol/L) | 16.36±3.96 | 19.23±3.67 | -2.289 | 0.028* |
| Ferritin | (ug/dl) | 2515±1937 | 3231±1938 | -1.07 | 0.292 |
| Dimensions of systolic function | LVEDD (mm/M2) | 39.36±5.79 | 42.73±6.39 | 1.716 | 0.094 |
| LVESD (mm/M2) | 24.52±3.91 | 26.87±5.93 | 1.512 | 0.139 |
| LVEF % | 67.96±5.55 | 67.20±9.21 | 0.327 | 0.746 |
| FS% | 38.40±4.32 | 37.47±7.61 | 0.497 | 0.622 |
| Dimensions of diastolic function | E wave (cm/sec) | 114.12±19.80 | 115.87±21.40 | 0.262 | 0.795 |
| A wave (cm/sec) | 56.16±15.40 | 58.53±24.20 | 0.380 | 0.706 |
| E/A ratio | 2.14±0.59 | 2.29±0.83 | 0.643 | 0.524 |
| Tei LV | 0.30±0.39 | 0.28±0.13 | 0.183 | 0.856 |
| Pulmonary artery systolic pressure | 37.16±8.27 | 38.93±10.92 | 0.570 | 0.572 |
*significant; TSH- Thyroid simulating hormone; FT4- Free thyroxin; LVEDD- Left ventricle end diastolic diameter; LVESD- Left ventricle end systolic diameter; EF- Left ventricular ejection fraction. FS- Fractional shortening; E- Early diastolic flow velocity; A- Late diastolic flow velocity
βTM cases with serum ferritin level above (2500 ug/dL) showed significant prolangation in E wave (p-value=0.029) [Table/Fig-10].
Thyroid hormones and echo parameters among the βTM patients in relation to serum ferritin level.
| Parameter (Mean±SD) | Serum ferritin level (μg/dl) | t-test |
|---|
| <2500 | > 2500 | t | p-value |
|---|
| TSH | (uIU/ml) | 2.55±1.45 | 3.15±1.10 | 1.39 | 0.171 |
| FT4 | (p mol/L) | 16.97±4.06 | 17.74±4.06 | -0.582 | 0.564 |
| Dimensions of systolic function | LVEDD (mm/M2) | 39.54±5.95 | 42.24±6.20 | -1.38 | 0.176 |
| LVESD (mm/M2) | 24.5±3.76 | 26.71±5.43 | -1.146 | 0.151 |
| LVEF % | 68.04±5.61 | 67.10±7.91 | 0.399 | 0.691 |
| FS% | 38.5±4.62 | 37.43±6.34 | -0.607 | 0.548 |
| Dimensions of diastolic function | E wave (cm/sec) | 110.46±20.10 | 123.13±11.90 | -2.264 | 0.029* |
| A wave (cm/sec) | 54.29±14.76 | 61.19±23.77 | -1.134 | 0.264 |
| E/A ratio | 2.14±0.58 | 2.22±0.74 | -0.668 | 0.508 |
| Tei LV | 0.32±0.38 | 0.24±0.12 | -0.825 | 0.414 |
| Pulmonary artery systolic pressure | 36.58±8.59 | 39.4±9.13 | -1.060 | 0.296 |
*significant; TSH- Thyroid simulating hormone; FT4- Free thyroxin; LVEDD- Left ventricle end diastolic diameter; LVESD- Left ventricle end systolic diameter; EF- Left ventricular ejection fraction; FS- Fractional shortening; E- Early diastolic flow velocity; A- Late diastolic flow velocity
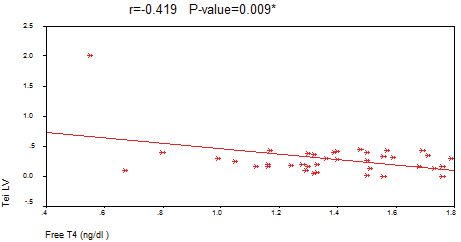
Spearman’s correlation in βTM patients; FT4 showed significant negative correlation with TSH and Tei LV, whereas ferritin showed significant positive correlation with TSH, LVEDD and LVESD [Table/Fig-11,12] .
Correlation between thyroid hormones, ferritin and echo parameters among the βTM patients.
| βTM cases parameters | TSH | Free T4 | Ferritin |
|---|
| r | p-value | r | p-value | r | p-value |
|---|
| TSH | | - | - | -0.3345 | 0.0349* | 0.3498 | 0.0269* |
| Free T4 | | -0.3345 | 0.0349* | - | - | 0.0132 | 0.9356 |
| Ferritin | (ug/dl) | 0.280 | 0.0797 | -0.018 | 0.9171 | - | - |
| Dimensions of systolic function | LVEDD (mm/M2) | 0.059 | 0.716 | -0.064 | 0.694 | 0.3257 | 0.0403* |
| LVESD (mm/M2) | 0.108 | 0.508 | -0.025 | 0.877 | 0.3689 | 0.0192* |
| LVEF % | -0.095 | 0.562 | -0.050 | 0.761 | -0.151 | 0.3523 |
| FS% | -0.121 | 0.455 | -0.075 | 0.644 | -0.2082 | 0.1974 |
| Dimensions of diastolic function | E wave (cm/sec) | 0.094 | 0.564 | 0.082 | 0.616 | - | - |
| A wave (cm/sec) | 0.014 | 0.929 | -0.086 | 0.597 | - | - |
| E/A ratio | 0.130 | 0.425 | 0.119 | 0.466 | 0.02807 | 0.8635 |
| Tei LV | -0.186 | 0.265 | -0.419 | 0.009* | -0.0251 | 0.8781 |
| Pulmonary artery systolic pressure | 0.239 | 0.143 | -0.107 | 0.516 | 0.1598 | 0.3247 |
*significant; TSH- Thyroid simulating hormone; FT4- Free thyroxin; LVEDD- Left ventricle end diastolic diameter; LVESD- Left ventricle end systolic diameter; EF- Left ventricular ejection fraction. FS- Fractional shortening; E wave- Early diastolic flow velocity; A wave- Late diastolic flow velocity
Correlation between FT4 and the myocardial performance index (Tei LV) among the βTM cases.
Discussion
Though iron chelating therapy has improved outcomes noticeably, cardiac failure remains an important cause of death in βTM [11]. Various pathologies were proposed including chronic anaemia, tissue hypoxia, myocarditis and iron overload, with subsequent diastolic then systolic dysfunction. Moreover; iron overload in the lungs leading to elevated pulmonary resistance, RV dilatation and dysfunction result in heart failure [12].
In this study, 25% βTM had impaired thyroid function {primary (n=1, 2.5%); subclinical (n=5, 12.5%); and central (secondary) hypothyroidism (n=4, 10%)}. The severity of endocrine disease was associated with an increase of ferritin levels. In βTM thyroid dysfunction was reported in variable prevalence in different cohorts [13-15]. This discrepancy was related to genetic, geographic, cultural, economic factors, methods used for thyroid function, ages, different amount and quality of blood transfusion, different medications, type and dosages of iron-chelating therapy.
In this study; heart rate was higher in βTM, in contrast Garadah TS and colleagues reported lower heart rate in βTM as single clinical manifestation of hypothyroidism [16], and Lala R and colleagues found marked increase in ferritin in non-splenectomized cases [17]. Belhoul KM and coworkers stated that endocrinopathy is common in βTM patients either with high serum ferritin levels or those splenectomized, however; we detect non-significant correlation between splenectomy and hypothyroidism [18].
The mean patient’s age was (9.51±3.26) and hypothyroidism is likely to be found in older age [19]. In contrast, Gulati R and colleagues reported the probability of endocrine dysfunction at an earlier age in developing countries [20].
In this study; LV dimensions (LVEDD and LVESD) were significantly higher in βTM but without changes in LVFS or EF% that indicated preserved systolic function; this was in agreement with [16,21-23]. These changes were evident in cases with older age, higher serum ferritin and longer duration of transfusion therapy; this was consistent with previous studies [24,25].
Moreover, Garadah TS and colleagues revealed a restrictive LV diastolic pattern with preserved LV systolic function [16].
De Sanctis V and colleagues established that cardiac abnormalities were common in βTM patients with severe iron overload and poor compliance to DFX and an improvement of LVEF was observed after either an intensive chelation therapy or thyroxin replacement therapy with regular iron chelation therapy [14].
In this study, EF% was significantly shorter in βTM cases with impaired thyroid function compared to cases with euthyroid function. However, the overall normal EF% indicates that βTM patients had minimal deleterious effect of iron overload on myocardial systolic function in spite of high ferritin level. This may also explain the absence of heart failure or significant valve diseases in the current study population. In contrast, Magri D and colleagues detected an impairment of myocardial function in βTM patients even in young, asymptomatic and well-chelated cases [24].
In this study, βTM cases had significantly higher peak flow velocity in early diastole (E-wave); while late (atrial or A-wave) peaks of flow velocity and the ratio between the early and late (atrial) peaks of flow velocity showed insignificant changes. Tei LV and pulmonary artery pressure were significantly increased in βTM cases with impaired thyroid function, age above (nine years) and high serum ferritin. These results were in line with a study by Gulati R et al., [20] and partially consistent with Kremastinos DT et al., [26] who revealed altered diastolic function by an increase of both early and late peak trans-mitral flow velocity without change of the E/A ratio.
In contrast, other studies [9,27,28] reported an increase in peak flow velocity at early diastole E-wave and the E/A ratio in βTM cases, while Yaprak and colleagues revealed significant higher E-wave, E/A ratio, and lower A-wave velocity suggestive of a restrictive pattern [29].
In this study, Tei LV was significantly high in βTM cases with impaired thyroid function compared to controls or cases with euthyroid state, suggestive of early left ventricular dysfunction. This result was in line with Frommelt PC et al., [30].
In this study, the mean pulmonary pressure was insignificantly higher in βTM. In contrast Hahalis G and colleagues revealed pulmonary hypertension in βTM patient’s resulting from pulmonary haemosiderosis [11].
In this study, we found insignificant difference in systolic and diastolic functions in βTM patients in relation to Hb level, this finding consistent with Yaprak I et al., [29].
Bosi G and colleagues found significant weak negative correlation between LVEF% and serum ferritin and he reported that the overall cardiovascular prognosis was good if the serum ferritin is below 2500 ng/dL. The higher mean age of the patients in Bosi’s study might be the responsible for the detection of LV systolic dysfunction [27].
However, Iarussi D reported right sided heart failure in 16% of βTM patients with high serum ferritin (>2500 ng/mL) [31,36]. Also, Wood JC and colleagues found that cardiac risk is conveyed by positive iron balance over a prolonged period of time [25].
In this study, we found non-significant impairment in diastolic function parameters. In contrast, Silvilairat S and colleagues found that LV diastolic dysfunction was correlated to serum ferritin as LV diastolic dysfunction was absent in patients with serum ferritin (<2500 ng/mL) and was present in patients with serum ferritin (>5000 ng/mL) [32]. It seems that myocardial disease goes through an impaired relaxation stage before development of systolic dysfunction. This maybe due to iron overload and increased stiffness of the LV wall [9].
In the current study, although all βTM cases documented receiving iron chelation therapy, serum ferritin was more than 35 times higher in the βTM cases compared with controls, therefore, the iron burden was severe, indicating suboptimal dosage and/or compliance. These finding confirms that iron overload mediate the impaired diastolic function leading to stiffness of the myocardial wall but with well-preserved LV systolic function, this consistent with Aydinok Y et al., and Garadah T et al., [5,33].
In this study, significance positive correlation between ferritin and TSH, LVEDD and LVESD was established and significance negative correlation between FT4 with TSH and myocardial performance index (Tei LV) was found.
Limitation
The small sample size used is the major limitation of the current study. Therefore, it is considered a pilot study and further studies on a larger cohort are warranted.
Conclusion
Improper chelation therapy results in cardiac dysfunction and a considerable proportion have thyroid dysfunction, therefore, all transfusion dependent βTM children needs optimal early use of chelation therapy, wise blood transfusion and periodic evaluation of thyroid and cardiac functions. Thyroxin replacement therapy may be considered in βTM cases having combined iron overloaded and subclinical hypothyroidism or cases with poor response to chelation therapy.
Hb: Haemoglobin; TSH: Thyroid simulating hormone; FT4: Free thyroxinand
TSH: Thyroid simulating hormone; FT4: Free thyroxin
*significant; Head circumference: HC TSH: Thyroid simulating hormone; FT4: Free thyroxin; LVEDD: Left ventricle end diastolic diameter; LVESD: Left ventricle end systolic diameter; LVEF: Left ventricular ejection fraction; FS: Fractional shortening; E wave: Early diastolic flow velocity; A wave: Late diastolic flow velocity
*significant; a: impaired vs. control; b: normal function vs. control; c: impaired vs. normal function)TSH: Thyroid simulating hormone; FT4: Free thyroxin; LVEDD: Left ventricle end diastolic diameter; LVESD: Left ventricle end systolic diameter; LVEF: Left ventricular ejection fraction; FS: Fractional shortening; E wave: Early diastolic flow velocity; A wave: Late diastolic flow velocity
*significant; TSH- Thyroid simulating hormone; FT4- Free thyroxin; LVEDD- Left ventricle end diastolic diameter; LVESD- Left ventricle end systolic diameter; LVEF- Left ventricular ejection fraction; FS- Fractional shortening; E- Early diastolic flow velocity; A- Late diastolic flow velocity
*significant; TSH- Thyroid simulating hormone; FT4, Free thyroxin; LVEDD- Left ventricle end diastolic diameter; LVESD- Left ventricle end systolic diameter; LVEF- Left ventricular ejection fraction; FS- Fractional shortening; E- Early diastolic flow velocity; A- Late diastolic flow velocity
TSH-Thyroid simulating hormone; FT4-Free thyroxin; LVEDD-Left ventricle end diastolic diameter; LVESD-Left ventricle end systolic diameter; LVEF- Left ventricular ejection fraction; FS- Fractional shortening; E- Early diastolic flow velocity; A- Late diastolic flow velocity
TSH-Thyroid simulating hormone; FT4-Free thyroxin; LVEDD-Left ventricle end diastolic diameter; LVESD- Left ventricle end systolic diameter; LVEF- Left ventricular ejection fraction; FS- Fractional shortening; E- Early diastolic flow velocity; A- Late diastolic flow velocity
*significant; TSH- Thyroid simulating hormone; FT4- Free thyroxin; LVEDD- Left ventricle end diastolic diameter; LVESD- Left ventricle end systolic diameter; EF- Left ventricular ejection fraction. FS- Fractional shortening; E- Early diastolic flow velocity; A- Late diastolic flow velocity
*significant; TSH- Thyroid simulating hormone; FT4- Free thyroxin; LVEDD- Left ventricle end diastolic diameter; LVESD- Left ventricle end systolic diameter; EF- Left ventricular ejection fraction; FS- Fractional shortening; E- Early diastolic flow velocity; A- Late diastolic flow velocity
*significant; TSH- Thyroid simulating hormone; FT4- Free thyroxin; LVEDD- Left ventricle end diastolic diameter; LVESD- Left ventricle end systolic diameter; EF- Left ventricular ejection fraction. FS- Fractional shortening; E wave- Early diastolic flow velocity; A wave- Late diastolic flow velocity
[1]. El-Beshlawy A, Youssry I, Prevention of haemoglobinopathies in EgyptHaemoglobin 2009 22:S14-20.10.3109/0363026090334639520001619 [Google Scholar] [CrossRef] [PubMed]
[2]. Herbert L, Muncie JR, James S, Campbell M, Alpha and beta thalassaemiaAm Fam Physician 2009 80(4):339-44. [Google Scholar]
[3]. Filosa A, Di Maio S, Aloj G, Acampora C, Longitudinal study on thyroid function in patients with thalassaemia majorJ Paediatr Endocrinol Metab 2006 19:1397-404.10.1515/JPEM.2006.19.12.139717252692 [Google Scholar] [CrossRef] [PubMed]
[4]. Thomas AS, Garbowski M, Ang AL, Shah FT, Walker AS, Moon JC, A decade follow-up of a thalassaemia major (TM) cohort monitored by Cardiac Magnetic Resonance imaging (CMR): significant reduction in patients with cardiac iron and in total mortalityBlood 2010 116(21):101110.1182/blood.V116.21.1011.1011 [Google Scholar] [CrossRef]
[5]. Aydinok Y, Porter JB, Piga A, Elalfy M, El-Beshlawy A, Kilinç Y, Prevalence and distribution of iron overload in patients with transfusion-dependent anaemias differs across geographic regions: results from the CORDELIA studyEur J Haematol 2015 95(3):244-53.10.1111/ejh.1248725418187 [Google Scholar] [CrossRef] [PubMed]
[6]. Tsironi M, Korovesis K, Farmakis D, Deftereos S, Aessopos A, Hypocalcemic heart failure in thalassemic patientsInt J Hematol 2006 83:314-17.10.1532/IJH97.E053216757430 [Google Scholar] [CrossRef] [PubMed]
[7]. Aessopos A, Berdoukas V, Tsironi M, The heart in transfusion dependent homozygous thalassaemia today- prediction, prevention and managementJournal compilation 2008 80:93-106.10.1111/j.1600-0609.2007.01018.x18081719 [Google Scholar] [CrossRef] [PubMed]
[8]. Abdulzahra MS, Al-Hakeim HK, Ridha MM, Study of the effect of iron overload on the function of endocrine glands in male thalassaemia patientsAsian Journal of Transfusion Science 1999 52(1):75-772.10.4103/0973-6247.8323621897589 [Google Scholar] [CrossRef] [PubMed]
[9]. Gharzuddine WS, Kazma HK, Nuwayhid IA, Bitar FF, Koussa SF, Moukarbel GV, Doppler characterization of left ventricular diastolic function in beta-thalassaemia major. Evidence for an early stage of impaired relaxationEur J Echocardiogr 2002 3(1):47-51.10.1053/euje.2001.011412067534 [Google Scholar] [CrossRef] [PubMed]
[10]. Aessopos A, Deftereos S, Tsironi M, Karabatsos F, Yousef J, Fragodimitri C, Predictive echo-Doppler indices of left ventricular impairment in β-thalassemic patientsAnn Hematol 2007 86(1):429-34.10.1007/s00277-007-0257-y17262191 [Google Scholar] [CrossRef] [PubMed]
[11]. Hahalis G, Alexopoulos D, Kremastinos DT, Zoumbos NC, Heart failure in beta-thalassaemia syndromes: a decade of progressAm J Med 2005 118:957-67.10.1016/j.amjmed.2005.02.02116164878 [Google Scholar] [CrossRef] [PubMed]
[12]. Hahalis G, Manolis AS, Apostolopoulos D, Alexopoulos D, Vagenakis AG, Right ventricular cardiomyopathy in beta-thalassaemia majorEur Heart J 2002 23(1):147-56.10.1053/euhj.2001.270911785997 [Google Scholar] [CrossRef] [PubMed]
[13]. Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, Survival and complications in patients with thalassaemia major treated with transfusion and deferoxamineHaematologica 2004 89(10):1187-93. [Google Scholar]
[14]. De Sanctis V, De Sanctis E, Ricchieri P, Gubellini E, Gilli G, Gamberini MR, Mild subclinical hypothyroidism in thalassaemia major: prevalence, multigated radionuclide test, clinical and laboratory long-term follow-up studyPediatr Endocrinol Rev 2008 6(Suppl 1):174-80. [Google Scholar]
[15]. Abdel-Razek AR, Abdel-Salam A, El-Sonbaty MM, Youness ER, Study of thyroid function in Egyptian children with β-thalassaemia major and β-thalassaemia intermediaJ Egypt Public Health Assoc 2013 88(3):148-52.10.1097/01.EPX.0000436490.10201.2824374948 [Google Scholar] [CrossRef] [PubMed]
[16]. Garadah TS, Mahdi NA, Jaradat AM, Hasan ZA, Nagalla DS, Thyroid function [16]status and echocardiographic abnormalities in patients with beta thalassaemia major in BahrainCardiology 2013 7:21-27.10.4137/CMC.S1070223400522 [Google Scholar] [CrossRef] [PubMed]
[17]. Lala R, Chiabotto P, Di Stefano M, Isaia GC, Garofalo F, Piga A, Bone density and metabolism in thalassaemiaJ Pediatr Endocrinol Metab 2005 11:785-90. [Google Scholar]
[18]. Belhoul KM, Bakir ML, Saned MS, Kadhim AM, Musallam KM, Taher AT, Serum ferritin levels and endocrinopathy in medically treated patients with β thalassaemia majorAnn Hematol 2012 91(7):1107-14.10.1007/s00277-012-1412-722281991 [Google Scholar] [CrossRef] [PubMed]
[19]. Abdalla PA, Fawzi PA, Salem PA, El-Banna PA, Tayyem C, Ahmad J, Increased oxidative stress and iron overload in jordanian β-thaassemic childrenHaemoglobin 2011 35:67-79.10.3109/03630269.2010.54462421250883 [Google Scholar] [CrossRef] [PubMed]
[20]. Gulati R, Bhatia V, Agarwal SS, Early onset of endocrine abnormalities in beta-thalassaemia major in a developing countryJ Pediatr Endocrinol Metab 2000 13(6):651-56.10.1515/JPEM.2000.13.6.65110905390 [Google Scholar] [CrossRef] [PubMed]
[21]. Khalifa AS, Ayoub AM, Al-Shabrawy L, Cardiac changes in Beta-thalassaemia major and effect of treatmentEgypt J Ped 1989 6:415 [Google Scholar]
[22]. Desideri A, Scatolin G, Gobellini A, Cavuto F, Left ventricular function in thalassaemia major: protective effect of desferoxamineCan J Cardiol 1994 10(1):63-66. [Google Scholar]
[23]. Hui L, Leung MP, Ha SY, Chau AK, Cheung YF, Early left ventricular dysfunction and chelation therapy in thalassaemia majorHeart 2003 89:669-70.10.1136/heart.89.6.66912748234 [Google Scholar] [CrossRef] [PubMed]
[24]. Magrì D, Sciomer S, Fedele F, Gualdi G, Sciomer E, Pugliese P, Early impairment of myocardial function in young patients with beta-thalassaemia majorEur J Haematol 2008 80(6):515-22.10.1111/j.1600-0609.2008.01054.x18284626 [Google Scholar] [CrossRef] [PubMed]
[25]. Wood JC, Cardiac iron across different transfusion-dependent diseasesBlood Rev 2008 22:14-21.10.1016/S0268-960X(08)70004-3 [Google Scholar] [CrossRef]
[26]. Kremastinos DT, Tsiapras DP, Tsetsos GA, Rentoukas EI, Vretou HP, Toutouzas PK, Left ventricular diastolic Doppler characteristics in Thalassaemia MajorCirculation 1993 88:1127-35.10.1161/01.CIR.88.3.11278353874 [Google Scholar] [CrossRef] [PubMed]
[27]. Bosi G, Crepaz R, Gamberini MR, Fortini M, Scarcia S, Bonsante E, Left ventricular remodeling and systolic and diastolic function in young adults with b thalassaemia major: a Doppler echocardiographic assessment and correlation with haematological dataHeart 2003 89(1):762-66.10.1136/heart.89.7.76212807852 [Google Scholar] [CrossRef] [PubMed]
[28]. Mamtani M, Kulkarni H, Influence of iron chelator on myocardial iron and cardiac function in transfusion dependent thalassaemia: a systematic review and meta-analysisBr J Haematol 2008 141:882-90.10.1111/j.1365-2141.2008.07122.x18355381 [Google Scholar] [CrossRef] [PubMed]
[29]. Yaprak I, Aksit S, Ozturk C, Bakiler AR, Dorak C, Turker M, Left ventricle diastolic abnormalities in children with beta-thalassaemia major: Doppler echocardiographic studyTurk J Pediatr 1988 40(2):201-19. [Google Scholar]
[30]. Frommelt PC, Ballweg JA, Whitstone BN, Frommelt MA, Usefulness of Doppler tissue imaging analysis of tricuspid annular motion for determination of right ventricular function in normal infants and childrenAm J Cardiol 2002 89:610-13.10.1016/S0002-9149(01)02308-6 [Google Scholar] [CrossRef]
[31]. Iarussi D, Di Salvo G, Pergola V, Coppolino P, Tedesco MA, Ratti G, Pulsed Doppler tissue imaging and myocardial function in thalassaemia majorHeart Vessels 2003 18:1-6.10.1007/s00380030000012644874 [Google Scholar] [CrossRef] [PubMed]
[32]. Silvilairat S, Sittiwangkul R, Pongprot Y, Charoenkwan P, Phornphutkul C, Tissue Doppler echocardiography reliably reflects severity of iron overload in paediatric patients with b thalassaemiaEur J Echocardiogr 2008 9:368-72.10.1016/j.euje.2007.06.00317689292 [Google Scholar] [CrossRef] [PubMed]
[33]. Garadah T, Kassab S, Mahdi N, Golan A, Abu-Taleb A, Jamsheer A, Pulsed and tissue Doppler echocardiographic changes in patients with thalassaemia majorClinical Medicine: Blood Disorders 2010 3:1-8.10.4137/CMBD.S4377 [Google Scholar] [CrossRef]