Typically, Renal Cell Carcinoma (RCC) presents with distinguishing features of flank pain, abdominal palpable mass and haematuria. Other clinical symptoms include weight loss, anorexia and metastasis to the lungs. In rare instances, patients may present with infected renal mass that may be underlying malignancy. Herein, we report a case of a 65-year-old, African American man presenting with flank pain and fever suspected of a renal abscess and eventually diagnosed with Papillary RCC (pRCC) type II requiring radical nephrectomy and initiating adjuvant tyrosine kinase inhibitor (sunitinib).
Radical Nephrectomy, Renal abscess, Sunitinib
Case Report
A 65-year-old, African American man who reported in good health until approximately three days before the admission, when he began to experience sharp pain in the bilateral lower quadrant radiating to the right flank. The pain worsened daily, with the development of brown urine and a fever of 101°F two days before admission. The evening before the admission, his fever continued to be as high as 101°F even after the administration of Tylenol. The patient had worked as a painter and a maintenance employee prior to his retirement three years ago.
On arrival, the patient reported severe abdominal pain but no dysuria, pyuria, foul-smelling urine, chills, or emesis. About one month ago, he had been treated with a one-week course of antibiotics for a urinary tract infection and had lost 3.5 kg body weight in the past three weeks.
On examination, the patient was alert and fully oriented. His temperature was recorded to be 101°F, blood pressure of 132/81 mmHg (value obtained via electronic BP instrument), pulse of 110 beats/minute, respiratory rate of 20 cycles/minute and oxygen saturation of 99% while breathing ambient air. The patient’s height was 180.34 cm, weight was 130.1 pounds, and body mass index was 18.30 kg/m2. There was a visible bulge near the right iliac fossa and normoactive bowel sounds with tenderness on palpation of the right lower quadrant and suprapubic area, with no rebound tenderness. Palpation revealed an approximately 20 cm × 16 cm, tender, globular mass
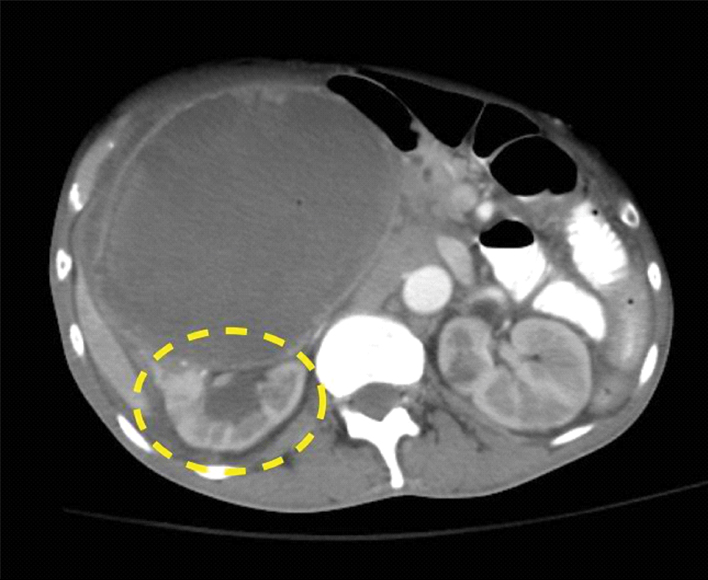
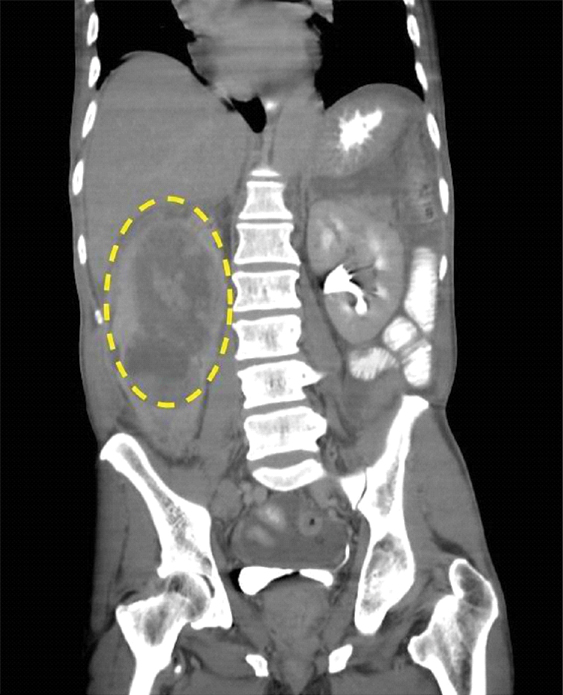
On admission, urinalysis revealed amber-colored urine with specific gravity of 1.020, pH of 6.0, positive ketones bodies, leukocyte esterase, nitrites, 11 Red Blood Cells (RBCs), excess White Blood Cells (WBCs), and the presence of many clumps of bacteria. A complete blood count revealed WBC 21,000 k/mm3; 90% segmental neutrophils; haematocrit 25.9%; haemoglobin 8.1 gm/dL; mean corpuscular volume 72.2 μ3; and platelets, 982 k/mm3. Laboratory values revealed blood urea nitrogen of 16 mg/dL; creatinine of 0.94 mg/dL; glomerular filtration rate of >60 mL/minutes/1.73 m2; lactic acid 2.2 mmol/L; and albumin of 2.8 gm/dL. Inflammatory markers were as follows: erythrocyte sedimentation rate 128 mm/hour and C-reactive protein 200 mg/L. Contrast-enhanced CT of the abdomen revealed a large, complex mass impinging on the right peritoneal cavity and exhibiting a claw sign demonstrating origination within the right kidney. Tiny pockets of gas were visible within the mass which raised the suspicion of a superimposed infection [Table/Fig-1]. The mass was 13.5 cm in the anteroposterior dimension, 15.2 cm in the mediolateral dimension, and 21.9 cm in the cephalocaudal dimension [Table/Fig-2]. With the nature of presentation, our provisional diagnosis for this patient was renal abscess. Therefore, he was started with administration of normal saline, intravenous broad-spectrum antibiotics (vancomycin 1 gm every 12 hours and piperacillin-tazobactam 3.375 gm every 8 hours), and pain medication was initiated.
Right kidney with visible gas-filled pockets and a mass with highlighted claw sign indicating its renal origin.
Erect view of the right kidney encompassed by a mass of 13.5 cm in the anteroposterior, 15.2 cm in the mediolateral, and 21.9 cm the cephalocaudal dimensions.
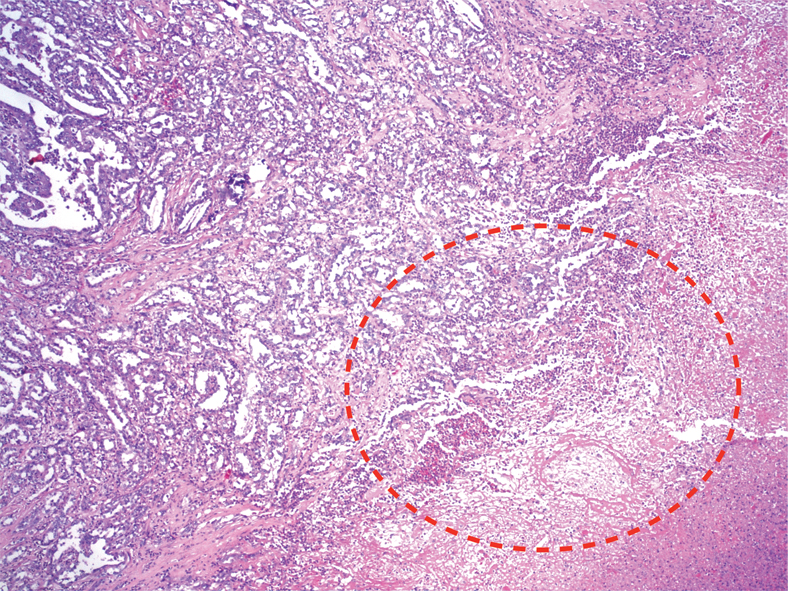
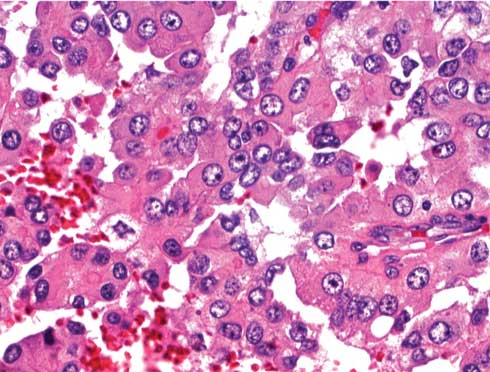
CT-guided Incision and Drainage (ID) with placement of a Jackson-Pratt drain yielded 200 mL of purulent, foul-smelling fluid. Despite intravenous antibiotics, the patient’s haemoglobin decreased to 7 gm/dL with leukocytosis (27 k/mm3). Abdominal pain and fever persisted, and on Day 2, blood and urine cultures tested positive for Escherichia coli. On Day 4, abdominal pain and fever continued despite antibiotic administration and drainage. Drainage fluid cultures showed E. coli growth. Cytology was positive for inflammatory cells, including neutrophils, blood, and fibrin. Repeat CT on Day 7 showed no change in the size of the mass. Repeat ID on Day 9 yielded 1000 mL of necrotic and purulent fluid, and cytological evaluation revealed fragments of viable tumour consistent with the findings of pRCC. Histopathology of the tumour showed papillae surrounded by the cells with abundant granular cytoplasm, and prominent nucleoli associated with areas of necrosis. After ID, an abdominal CT revealed a 50% reduction in the size of the mass. A radical nephrectomy involving resection of a 17 cm tumour attached to the inferior vena cava was performed. The pathological diagnosis was pRCC with necrotic tumour extending to more than 50% of the perinephric fat (Biopsy [Table/Fig-3,4 and Table/Fig-5]). The patient was started on sunitinib treatment in the outpatient clinic. Within 10 days, he could not tolerate sunitinib treatment requiring admission to the hospital, and uniform decision was made for palliative care after consultation with his family. Patient passed away about 30 days from the time of discharge.
Biopsy specimen with areas of necrosis and abscess formation circled in red (Hematoxylin and Eosin, original magnification 20X).
Biopsy specimen of a viable tumor consistent with a papillary renal cell carcinoma; Hematoxylin and Eosin, original magnification (40X).
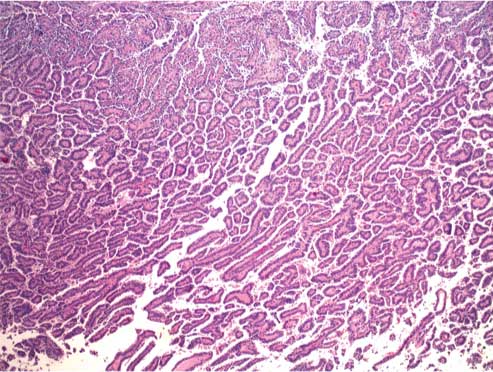
Biopsy specimen of a viable tumor consistent with a papillary renal cell carcinoma; Hematoxylin and Eosin, original magnification (400X).
Discussion
A continual increase in the incidental diagnosis of RCC can be attributed to advancements in diagnostic modalities due to which the number of cases diagnosed based on the classical triad of haematuria, flank pain, and abdominal mass has reduced [1]. Depending on the histology of RCC, there are two subtypes: "Clear" and "Papillary", of which pRCC is second most common accounting for 15-20% and is further classified as Type 1 and Type 2 [2,3]. Histologically, Type 1 consists of papillae and tubular structures shadowed with pale cytoplasm and small oval nuclei, while Type 2 is seen as papillae covered with large cells containing eosinophilic cytoplasm and large spherical nuclei with prominent nucleoli [2,4]. In 62 cases examined by Amin MB et al., they had concluded pRCC to be aggressive malignant tumour presenting in earlier stage that progresses rapidly [5], which was similar in presentation to our case with pRCC Type 2.
The differential diagnosis of patients presenting with flank pain, fever, and chills with an abdominal mass can be Xanthogranulomatous Pyelonephritis (XGP) or renal abscess. Eltahawy E et al., evaluated 11 patients within a 10-year period of which five patients had renal abscess and suspected renal mass was eventually diagnosed as RCC for two patients who had an initial negative biopsy [6]. XGP shares many characteristics with true renal neoplasms, including the radiological appearance and invasion in neighboring vasculature and organs [7]. Ultrasound does not differentiate between abscess and malignancy. CT is the diagnostic modality of choice for renal abscess, malignancy, and XGP as it provides excellent delineation of the tissue [7,8]. The amount of image distortion caused by infection superimposed on malignancy depends on the extent of tissue necrosis and breakdown and pus formation [8].
RCC treatment is guided by renal function, staging, extent of tumour spread. Surgical resection of the tumour is effective in cases of localised RCC and also is used as palliative treatment for metastatic disease [9]. The 2017 National Compressive Cancer Network (NCCN) guidelines recommend partial nephrectomy for patients with T1a tumours (tumour size <7 cm) are at high risk of developing chronic renal disease, or with multiple small or bilateral tumours [9]. Approximately, 5% of patients with RCC have inferior vena cava involvement. Radical nephrectomy is the generally preferred surgical procedure for treating localised RCC. When RCC involves inferior vena cava, it is required to completely remove the Gerota fascia with perifascial resection of the kidney, perirenal fat, regional lymph nodes and ipsilateral adrenal gland [9,10]. Compared to simple removal of the kidney, radical nephrectomy provides a better surgical margin because of the possible involvement of perinephric fat. About 20%-30% of patients with clinically localised disease develop metastatic disease after nephrectomy [11]. The NCCN guidelines recommend radical nephrectomy for such patients, who are also the standard of care for Stage II and III renal tumours [9]. In cases with tumour invasion in the renal vein and inferior vena cava, a well vascularised thrombus is covered with its own intimal surface [12]. If the inferior vena cava is involved, then it is clamped above and below the tumour thrombus and the thrombus is resected intact, with subsequent closure of the vena cava by end-to-end anastomosis. Patients with invasion in the inferior vena cava wall exhibit poor prognosis, despite aggressive surgery. The NCCN guidelines recommend lymph node dissection as a prognostic intervention for patients with enlarged lymph nodes, including those whose lymph nodes appear normal [9].
In early stage RCC, early adjuvant molecular therapy improves overall survival. The S-TRAC (Sunitinib Treatment of Renal Adjuvant Cancer) trial of patients at high risk of tumour recurrence after nephrectomy demonstrated the benefit of adjuvant therapy with sunitinib. The median duration of disease-free survival was significantly increased by a sunitinib regimen of 50 mg daily for four weeks on/two weeks off for one year following nephrectomy [13,14].
Conclusion
The association of renal tumours and perirenal abscesses is rare. RCC typically presents with haematuria, flank pain, and abdominal mass. It infrequently presents as a perinephric abscess with signs and symptoms of septic shock, particularly in the elderly, in patients with diabetes, or in patients who are immune compromised. In our patient, the extensive necrosis found in the surgical specimen was probably responsible for the development of perirenal collection, which ultimately brought the patient to clinical attention. We hope that this case helps to guide the diagnosis, management, and therapy of other rare presentations of RCC.
[1]. Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML, Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinomaAm J Surg Pathol 2003 27(5):612-24.10.1097/00000478-200305000-0000512717246 [Google Scholar] [CrossRef] [PubMed]
[2]. Chow WH, Dong LM, Devesa SS, Epidemiology and risk factors for kidney cancerNature Reviews Urology 2010 7(5):245-57.10.1038/nrurol.2010.4620448658 [Google Scholar] [CrossRef] [PubMed]
[3]. Linehan WM, Spellman PT, Ricketts CJ, Creighton CJ, Fei SS, Davis C, comprehensive molecular characterization of papillary renal cell carcinomaThe New England Journal of Medicine 2016 374(2):135-45.10.1056/NEJMoa150591726536169 [Google Scholar] [CrossRef] [PubMed]
[4]. Delahunt B, Eble JN, Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumoursMod Pathol 1997 10(6):537-44. [Google Scholar]
[5]. Amin MB, Corless CL, Renshaw AA, Tickoo SK, Kubus J, Schultz DS, Papillary (chromophil) renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 62 casesAm J Surg Pathol 1997 21(6):621-35.10.1097/00000478-199706000-000019199639 [Google Scholar] [CrossRef] [PubMed]
[6]. Eltahawy E, Kamel M, Ezzet M, Management of renal cell carcinoma presenting as inflammatory renal massUrol Ann 2015 7(3):330-33.10.4103/0974-7796.15205126229320 [Google Scholar] [CrossRef] [PubMed]
[7]. Chandanwale SS, Xanthogranulomatous pyelonephritis: unusual clinical presentation: a case report with literature reviewJ Family Med Prim Care 2013 2(4):396-98.10.4103/2249-4863.12394226664851 [Google Scholar] [CrossRef] [PubMed]
[8]. Perimenis P, Pyonephrosis and renal abscess associated with kidney tumoursBr J Urol 1991 68(5):463-65.10.1111/j.1464-410X.1991.tb15385.x1747718 [Google Scholar] [CrossRef] [PubMed]
[9]. Motzer RJ, Jonasch E, Agarwal N, Bhayani S, Bro WP, Chang SS, Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in OncololgyJ Natl Compr Canc Netw 2017 15(6):804-34.10.6004/jnccn.2017.010028596261 [Google Scholar] [CrossRef] [PubMed]
[10]. Lane BR, Canter DJ, Rini BI, Uzzo RG, Cancer of the Kidney. In: Devita VT Jr, Lawrence TS, Rosenberg SA, eds. DeVita, Hellman, and Rosenberg’s Principles and Practice of Oncology 2015 10th EditionPhiladelphiaPA: Wolters Kluwer Health:865-84. [Google Scholar]
[11]. Ravaud A, Motzer RJ, Pandha HS, George DJ, Pantuck AJ, Patel A, Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomyN Engl J Med 2016 375(23):2246-54.10.1056/NEJMoa161140627718781 [Google Scholar] [CrossRef] [PubMed]
[12]. Salam MA, Principles and Practice of Urology 2001 New DelhiJaypee Brothers Medical:c2013 [Google Scholar]
[13]. Jonasch E, Matin S, Pagliaro LC, Wood CG, Tannir NM, Renal Cell Carcinoma. In: Kantarjian HM, Wolff RA, Koller CA, eds. MD Anderson Manual of Medical Oncology 2011 2nd EditionNew YorkNY: McGraw-Hill:905-24. [Google Scholar]
[14]. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinomaJ Clin Oncol 2009 27(22):3584-90.10.1200/JCO.2008.20.129319487381 [Google Scholar] [CrossRef] [PubMed]