Non Lactational Breast Abscess due to Actinomyces odontolyticus: First Case Report from Asia
Dinoop Korol Ponnambath1, MS Priyadharshini2, Appalaraju Boppe3
1 Assistant Professor, Department of Microbiology, PSG Institute of Medical Sciences and Research, Coimbatore, Tamil Nadu, India.
2 Postgraduate Student, Department of Microbiology, PSG Institute of Medical Sciences and Research, Coimbatore, Tamil Nadu, India.
3 Professor and Head, Department of Microbiology, PSG Institute of Medical Sciences and Research, Coimbatore, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Dinoop Korol Ponnambath, Assistant Professor, Department of Microbiology, PSG Institute of Medical Sciences and Research, Coimbatore-641004, Tamil Nadu, India.
E-mail: drdinukp@gmail.com
Actinomycosis is a chronic infection caused by the aerotolerant anaerobe, Actinomyces spp., characterised by the formation of abscess, draining sinuses and tissue fibrosis. Primary actinomycosis of the breast is a rare clinical presentation. We describe a rare case of breast abscess due to Actinomyces odontolyticus in a 53-year-old premenopausal woman with invasive papillary carcinoma of breast. The organism was isolated from the breast abscess and the identity was confirmed using VITEK® 2 and Matrix Assisted Laser Desorption/Ionisation-Time of Flight (MALDI-TOF). The patient was managed with modified radical mastectomy and amoxicillin-clavulanic acid for six weeks. The patient recovered completely with no recurrence on follow up visits.
Actinomycosis, Breast infection, Carcinoma, Tissue fibrosis
Case Report
A 53-year-old premenopausal, non-diabetic, non-smoker female presented to the Surgery Outpatient Department (OPD) with complaints of a lump in the left breast with an ulcer in the areolar region with seropurulent discharge. The lump was initially small which gradually increased in size over the past one year. There was no history of fever, trauma, skin lesion, nipple piercing, previous antibiotic treatment, dental extraction or other surgical procedures. The patient had consented for further workup and investigations. Preliminary blood investigations were normal except for microcytic hypochromic anaemia (Haemoglobin-5.4 gm/dL, Haematocrit/Packed Cell Volume (PCV)-17.9%, Mean Corpuscular Volume (MCV)-65.2 fL, Mean Corpuscular Haemoglobin (MCH)-19.6 pg, Mean Corpuscular Haemoglobin Concentration (MCHC)-30 gm/ dL, Red cell Distribution Width (RDW)-20.3% and microcytic hypochromic RBC’s were observed in peripheral blood smear). Examination of the left breast revealed a 6×5 cm sized mobile, hard mass occupying the central and lower outer quadrant of the left breast with nipple erosion and retraction with serous discharge. Sonomammogram of the left breast revealed a large, lobulated heterogeneous lesion at 5-6 o’clock position which showed both solid and cystic components. A sinus tract was found to be extending from the lesion to a small cystic collection in the deeper plane indenting the pectoralis muscle. The Breast Imaging-Reporting and Data System (BI-RADS) categorisation of the sonomammogram was category 1 and was further suggested for Histopathological Examination (HPE) and correlation to rule out malignancy. Tru-cut biopsy and aspirated pus were sent for microbiological and HPE. Gram stained smear showed plenty of pus cells with Gram-positive cocci in clusters and thin filamentous Gram-positive bacilli with occasional branching[Table/Fig-1]. Culture on 5% sheep blood agar (aerobic and anaerobic-Anoxomat Mark II System, Massachusetts, USA) at 18 hours of incubation grew methicillin-sensitive Coagulase-Negative Staphylococcus (CoNS) and at 48-72 hours, heavy growth of aerotolerant anaerobic (better growth observed at anaerobic incubation) short Gram-positive bacilli was observed. The colonies on blood agar produced red pigmentation after one week of aerobic incubation[Table/Fig-2,3]. The isolate was non-motile, non-acid fast on Ziehl-Neelsen (ZN) staining and modified ZN staining. Biochemically, the isolate was positive for nitrate reduction and negative for catalase, oxidase, urease, aesculin, arginine and gelatin hydrolysis. The isolate was identified as Actinomyces odontolyticus (Excellent identification-98% probability) by VITEK® 2 ANC ID card (bioMerieux, Marcy-l’Etoile, France) and MALDI-TOF mass spectrometry with a score of 2.17 (MALDI Biotyper, Bruker Daltonics, Billerica, MA). HPE of the tru-cut biopsy was suggestive of invasive papillary carcinoma with the Immunohistochemistry (IHC) negative for Estrogen Receptor (ER), Progesterone Receptor (PR) and Her2/neu antigen expression with 40% Ki-67 proliferation index. The patient was managed with modified radical mastectomy of the left breast and empirical oral amoxicillin-clavulanic acid 625 mg (500 mg/125 mg) twice daily for six weeks duration. The patient improved symptomatically postoperatively and had no features of recurrent infection on follow up visits.
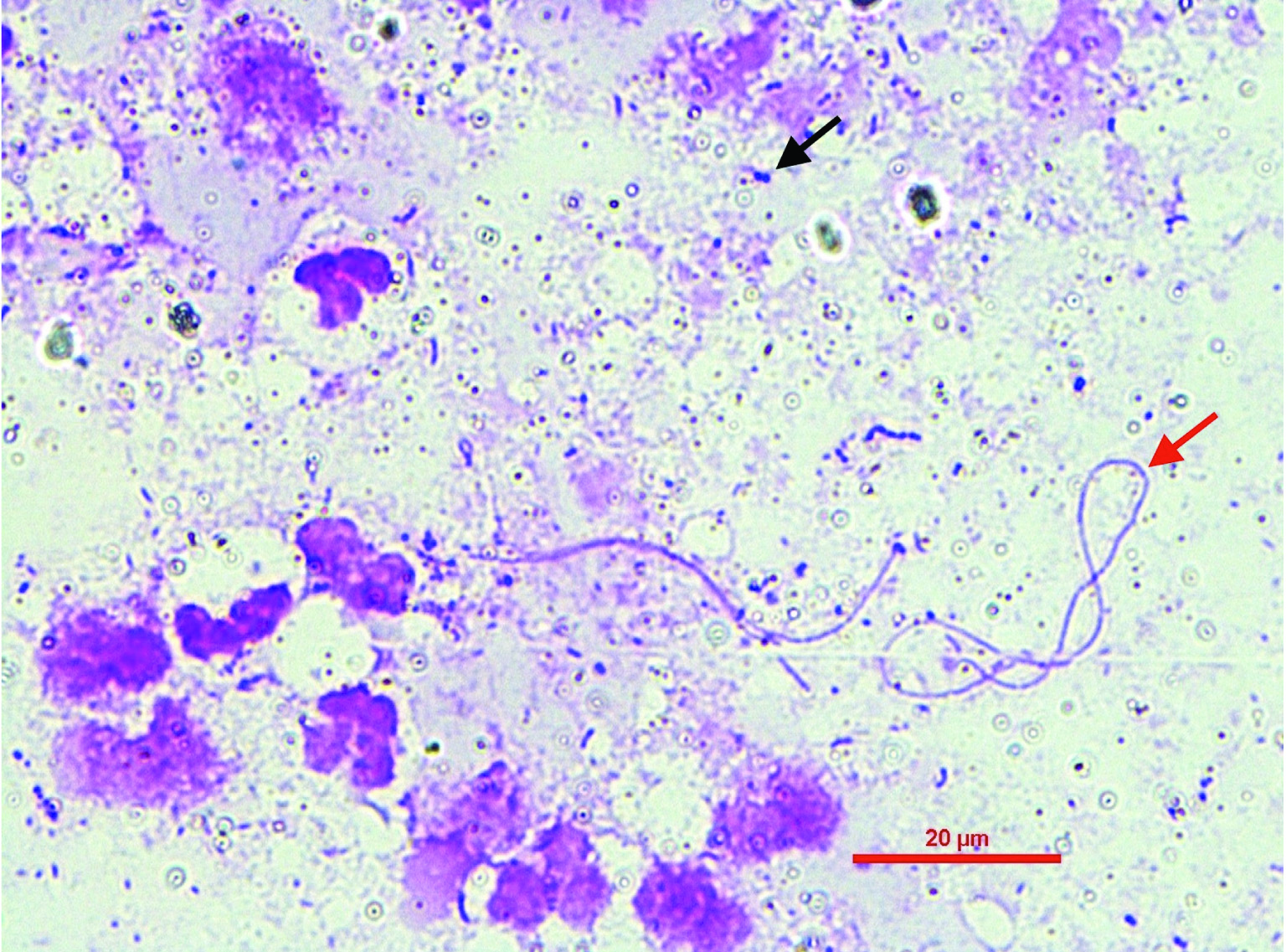
Gram staining of breast abscess pus showing pus cells with thin filamentous Gram-positive bacilli (pointed with red arrow) and Gram-positive cocci in clusters (pointed with black arrow) (Magnification -1000X).
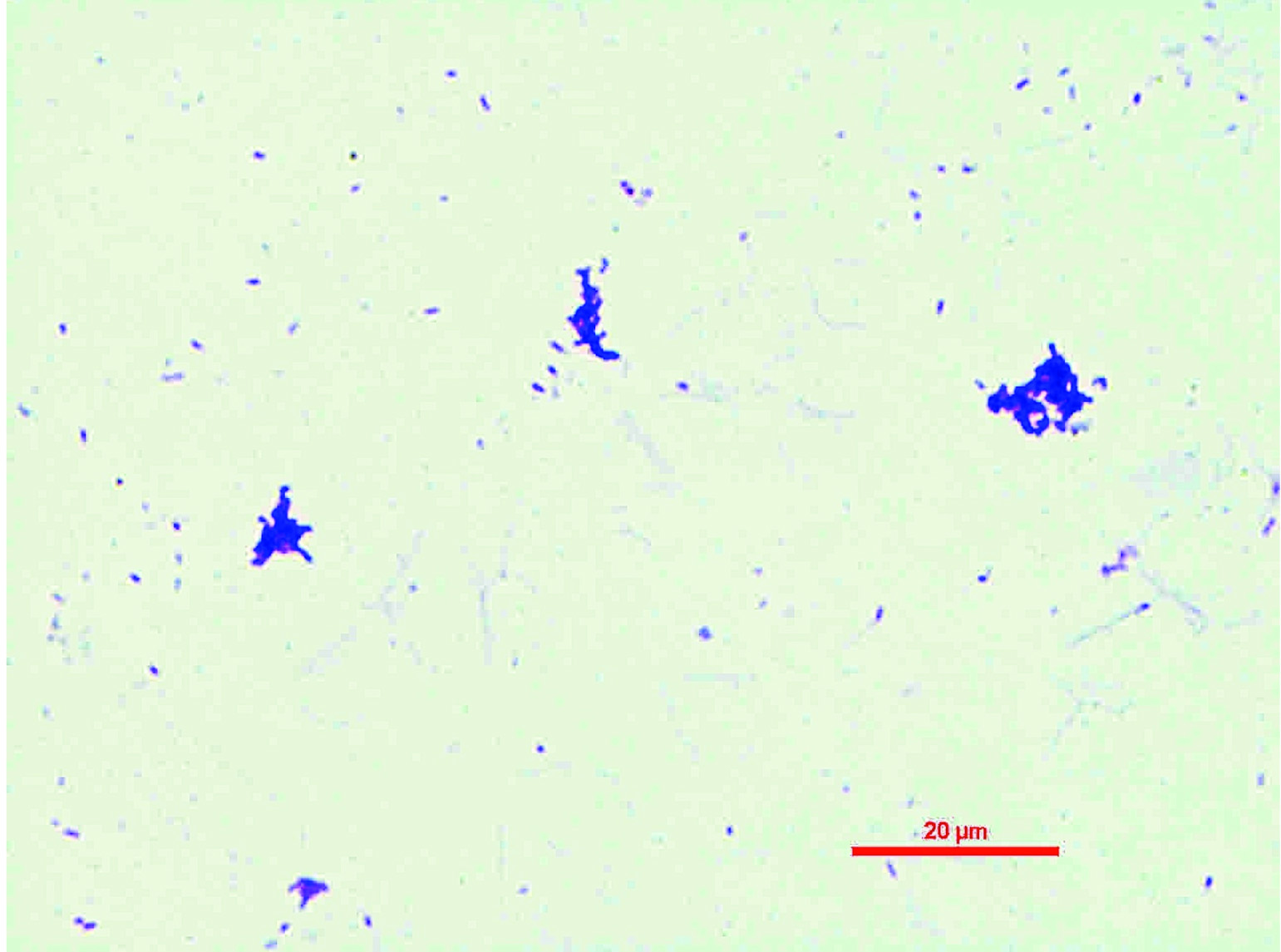
Gram staining of culture isolate from sheep blood agar showing Gram-positive rods (Magnification-1000X).
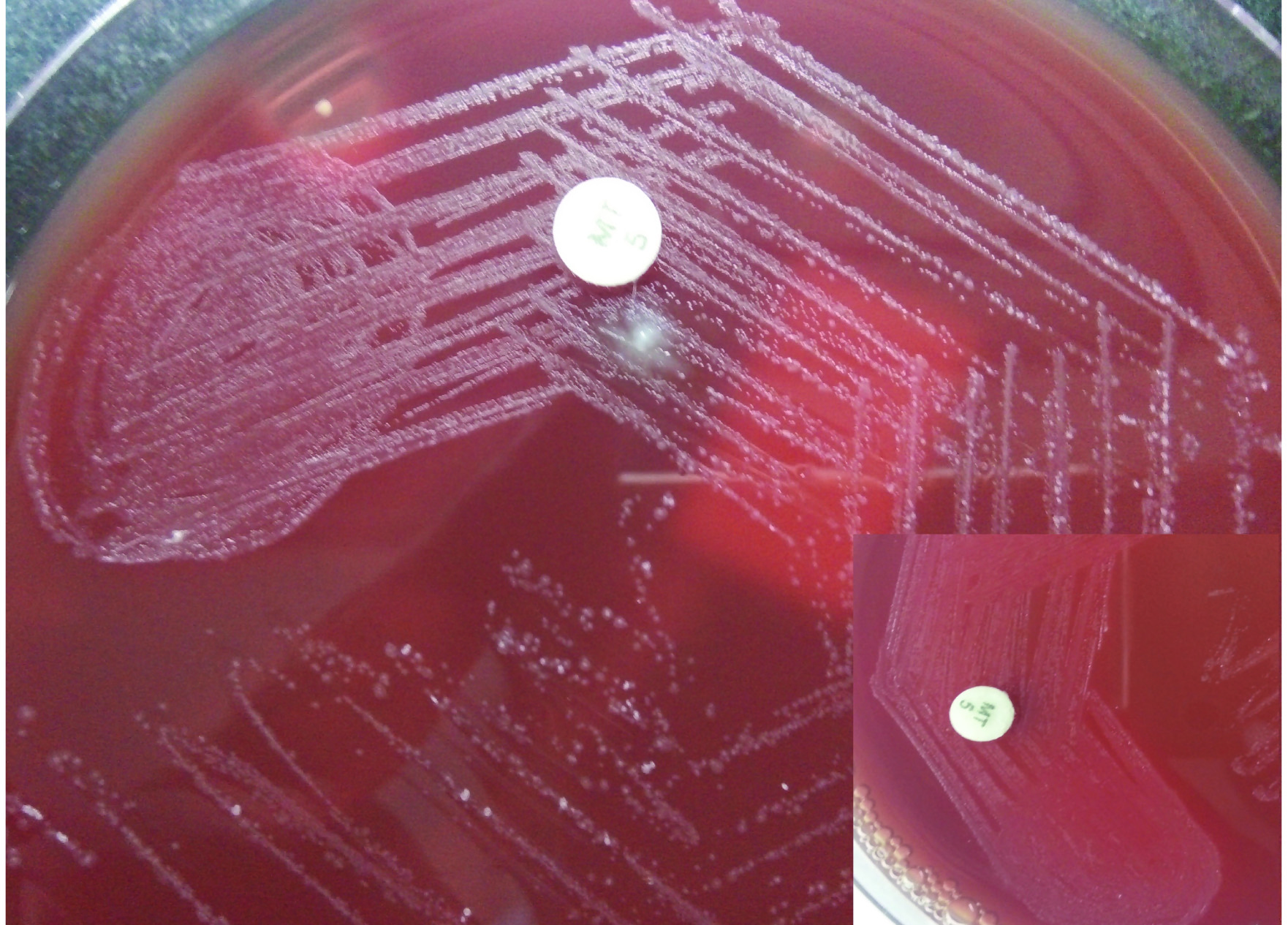
Growth of Actinomyces odontolyticus colonies (resistant to 5 μg metronidazole disc) on 5% anaerobic sheep blood agar showing occasional molar tooth appearance (Inner box-showing red pigmentation produced by colonies on anaerobic 5% sheep blood agar after 1 week of aerobic incubation).
Discussion
Actinomyces spp. is aerotolerant or anaerobic Gram-positive bacilli, which are usually isolated from the human mucosal surfaces (oropharynx, female genital tract, gastrointestinal tract). These organisms typically cause chronic sinus-forming lesions called actinomycosis and has been increasingly recognised to cause abscesses in various organs like liver, spleen, brain, breast etc., [1]. Breast is a rare site of primary actinomycotic infections and is usually classified as infections occurring in lactating or non lactating breasts [2]. Staphylococcus aureus remains the most common cause of breast abscess in lactating mothers while non lactating breast infections are complex due to their chronicity, atypical causative organisms (e.g., Corynebacterium/Actinomyces spp.) or polymicrobial nature and frequent recurrence [2]. Breast infections due to Actinomyces spp. can be primary or secondary. Primary actinomycosis of breast is common than secondary and are associated with risk factors like smoking, diabetes mellitus, obesity, nipple piercing procedures or trauma due to human bites [3]. In this case, none of these risk factors for primary actinomycosis were present and there was no other site of infection for secondary spread. Few authors have reported primary actinomycosis of breast occurring in diabetic postmenopausal women [3,4]. There are numerous reports of primary actinomycosis of breast presenting or masquerading as breast mass/malignancy [4-6]. Breast malignancy has not been described as a risk factor for primary actinomycosis in the literature while very few have reported the association of breast malignancy with actinomycosis of breast [7]. Earlier reports mentioned A. israelii as the most common species since most of these infections were diagnosed presumptively by Fine Needle Aspiration Cytology (FNAC) and HPE without culture confirmation [2,8]. Cases reported from India are shown in [Table/Fig-4][4,6,7,9-12]. Literature review revealed the newly described species, A. neuii as the most common Actinomyces species causing breast abscess followed by A. europaeus, A. turicensis and A. radingae [2,8]. There are very scarce reports of A. odontolyticus described as a causative agent of primary actinomycosis of breast. Review of published literature and reports of anaerobe reference unit reveals a total of 10 case reports of A. odontolyticus breast abscess [2]. Increased utilisation of newer identification methods like VITEK ID cards and MALDI-TOF has led to reliable identification and increased reporting of newer Actinomyces causing clinical infections.
Published cases of Actinomyces breast infections from India [4,6,7,9-12].
| S. no | Year | Age/Sex | Risk factor | Organism | Identification method | Treatment | Ref. no. |
|---|
| 1. | 1994 | 40/F | Nil | Culture not performed | FNAC and HPE | Antibiotics (IV Benzylpenicillin 12 MegaU OD-3 weeks followed by oral amoxicillin 500 mg tid-3 months) | [9] |
| 2. | 2012 | 50/F | Nil | A. israelii | Gram stain, culture and HPE | Information not available | [10] |
| 3. | 2012 | 32/F | Nil | Presumptively reported as A. israelii (Culture confirmation not performed) | FNAC and HPE | Antibiotics (Details not available) | [6] |
| 4. | 2012 | 61/F | Diabetes mellitus | Culture not performed | HPE | Antibiotics (Details not available) | [4] |
| 5. | 2014 | 55/F | Nil | Presumptively reported as A. israelii (Culture confirmation not performed) | HPE | Surgery (Modified radical mastectomy) | [11] |
| 6. | 2014 | 53/F | Invasive ductal carcinoma | Culture not performed | HPE Surgery | (Radical mastectomy) | [7] |
| 7. | 2016 | 25/F | Post-partum | Culture not performed | FNAC and HPE | Information not available | [12] |
Actinomycotic breast infections are usually treated with beta-lactam antibiotics since they are always susceptible to these groups of drugs. Beta-lactam inhibitor combinations like amoxicillin-clavulanic acid are usually preferred since these infections are polymicrobial, commonly co-infected with beta-lactamase producing organisms [13]. Drug susceptibility testing was not performed for this isolate due to non availability of CLSI M24-A2 guidelines and E-test strips for testing Actinomyces spp. Longer duration (2-6 weeks) of treatment is recommended for cure and prevention of recurrences, while surgical interventions may be required for extensive disease or recurrent disease [13].
Conclusion
Breast infections due to Actinomyces spp. are rare and are easily missed due to lack of clinical suspicion (masquerading as or rarely associated with malignancy), lack of improved diagnostic facilities and determining growth of coryneforms as a contaminant by microbiologists even when isolated from sterile sites. High index of suspicion with improved diagnostic facilities are keys to early diagnosis and treatment of this chronic relapsing infection.
[1]. Valour F, Sénéchal A, Dupieux C, Karsenty J, Lustig S, Breton P, Actinomycosis: etiology, clinical features, diagnosis, treatment, and managementInfect Drug Resist 2014 7:183-97.10.2147/IDR.S3960125045274 [Google Scholar] [CrossRef] [PubMed]
[2]. Bing AU, Loh SF, Morris T, Hughes H, Dixon JM, Helgason KO, Actinomyces species isolated from breast infectionsJ Clin Microbiol 2015 53(10):3247-55.10.1128/JCM.01030-1526224846 [Google Scholar] [CrossRef] [PubMed]
[3]. Gollapalli V, Liao J, Dudakovic A, Sugg SL, Scott-Conner CEH, Weigel RJ, Risk factors for development and recurrence of primary breast abscessesJ Am Coll Surg 2010 211(1):41-48.10.1016/j.jamcollsurg.2010.04.00720610247 [Google Scholar] [CrossRef] [PubMed]
[4]. Thambi R, Devi L, Sheeja S, Poothiode U, Primary breast Actinomyces simulating malignancy: a case diagnosed by fine-needle aspiration cytologyJ Cytol 2012 29(3):19710.4103/0970-9371.10117323112463 [Google Scholar] [CrossRef] [PubMed]
[5]. Salmasi A, Asgari M, Khodadadi N, Rezaee A, Primary actinomycosis of the breast presenting as a breast massbreast care 2010 5(2):105-07.10.1159/00030159920847823 [Google Scholar] [CrossRef] [PubMed]
[6]. Gupta C, Singh P, Bedi S, Kapur K, Primary actinomycosis of the breast masquerading as malignancy: diagnosis by fine needle aspiration cytologyBreast Care 2012 7(2):153-54.10.1159/00033777422740805 [Google Scholar] [CrossRef] [PubMed]
[7]. Suranagi V, Agrawal P, Bannur H, Hogade S, Actinomycosis of the breast: A rare association with breast carcinomaSaudi J Heal Sci 2014 3(1):5310.4103/2278-0521.130212 [Google Scholar] [CrossRef]
[8]. Attar KH, Waghorn D, Lyons M, Cunnick G, Rare Species of Actinomyces as Causative Pathogens in Breast AbscessBreast J 2007 13(5):501-05.10.1111/j.1524-4741.2007.00472.x17760673 [Google Scholar] [CrossRef] [PubMed]
[9]. Jain BK, Sehgal VN, Jagdish S, Ratnakar C, Smile SR, Primary actinomycosis of the breast: a clinical review and a case reportJ Dermatol 1994 21:497-500.10.1111/j.1346-8138.1994.tb01782.x8089371 [Google Scholar] [CrossRef] [PubMed]
[10]. Arora B, Giri S, Arora DR, Actinomycosis of breast-a case reportInt J Cur Res Rev 2012 4(20):85-88. [Google Scholar]
[11]. Chavan DR, Kullolli G, Metan BB, Javalgi AP, Primary Actinomycosis of Breast in Post-Menopausal Woman: A Case ReportJ Evolution Med Dent Sci 2014 3(19):5279-82.10.14260/jemds/2014/2588 [Google Scholar] [CrossRef]
[12]. Gosavi AV, Anvikar AR, Sulhyan KR, Manek DD, Primary actinomycosis of breast-A diagnosis on cytologyDiagn Cytopathol 2016 44(8):693-95.10.1002/dc.2349927238823 [Google Scholar] [CrossRef] [PubMed]
[13]. Smith AJ, Antimicrobial susceptibility testing of Actinomyces species with 12 antimicrobial agentsJ Antimicrob Chemother 2005 56(2):407-09.10.1093/jac/dki20615972310 [Google Scholar] [CrossRef] [PubMed]