Mammary Analogue Secretory Carcinoma- A Salivary Gland Neoplasm: Case Report of an Exceptionally Rare Tumour
Shailaja Shukla1, Anamika Kashyap2, Shuchita Sharma3, Lubaba Kamal4
1 Director Professor, Department of Pathology, Lady Hardinge Medical College, New Delhi, Delhi, India.
2 Senior Resident, Department of Pathology, Lady Hardinge Medical College, New Delhi, Delhi, India.
3 Postgraduate Student, Department of Pathology, Lady Hardinge Medical College, New Delhi, Delhi, India.
4 Postgraduate Student, Department of Pathology, Lady Hardinge Medical College, New Delhi, Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Anamika Kashyap, Senior Resident, Department of Pathology, Lady Hardinge Medical College, New Delhi-110001, Delhi, India.
E-mail: anamikakashyap25@gmail.com
Mammary Analogue Secretory Carcinoma (MASC) is a rare salivary gland tumour. It clinically presents as a painless mass in the parotid gland affecting mainly males. A 17-year-old male presented with 18 month history of a painless swelling of left parotid region. Physical examination showed a 3×3 cm, firm, non tender mass in the left parotid region. FNAC diagnosis of mucoepidermoid carcinoma was made. Final diagnosis of MASC was made on the basis of characteristic histopathology and immunohistochemistry. This tumour is considered as a low-grade carcinoma with a potential for high-grade transformation.
Mucin, Mucoepidermoid, Parotid
Case Report
A 17-year-old male presented to surgery department with an 18 month history of left parotid swelling which was painless and gradually increasing in size. There was no other complaint or any significant medical or family history. On examination, there was a 3×3 cm firm, non tender, mobile swelling in left parotid region with unremarkable overlying skin. No lymph nodes were clinically palpable. No facial nerve weakness was present. Ultrasonography and MRI revealed a heterogeneously hypoechoic, well-defined lesion in superficial lobe of left parotid gland and a clinicoradiological diagnosis of parotid tumour was made.
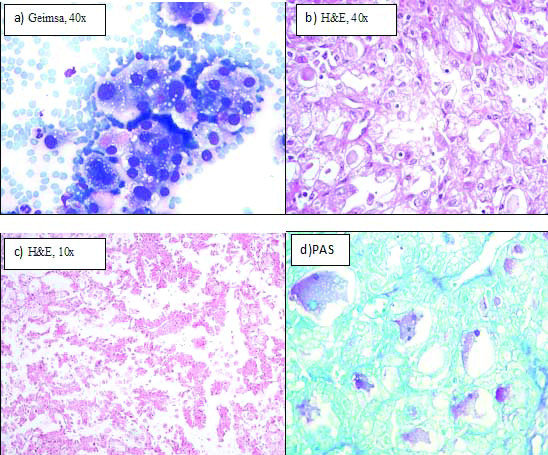
FNAC of the swelling showed tumour cells to have granular to vacuolated cytoplasm with presence of extracellular mucin in the background [Table/Fig-1a]. A provisional diagnosis of mucoepidermoid carcinoma was given on the basis of these findings and excision was advised. Intraoperatively, a 3×3 cm mass involving superficial lobe of parotid was noticed and removed withclear margins preserving the facial nerve. Superficial lobe of left parotid with the tumour along-with part of deep lobe of parotid was sent for histopathology. Grossly, tumour was well-circumscribed and unencapsulated with firm consistency and grey-white cut surface. Microscopically, tumour showed solid, cystic, tubular and papillary architecture with individual cell having abundant eosinophilic granular to vacuolated cytoplasm and vesicular nuclei [Table/Fig-1b,c]. There was presence of intraluminal and intracellular mucin that stained positive with alcian blue, PAS (Fast green as counterstain) and mucicarmine [Table/Fig-1d]. Mitotic figures were rare with absence of necrosis and perineural invasion. No squamoid differentiation was seen.
a) Fine needle aspiration smear showing cells with pink granular to vacuolated cytoplasm (Geimsa, 40X); b) Tumour showing tubular pattern with intraluminal eosinophilic colloid-like secretions, cells with eosinophilic granular cytoplasm and vesicular nucleus (H&E, 40X); c) Papillary architecture (H&E, 10X); d) PAS positive magenta coloured intraluminal mucin (Fast green as counterstain; 40X).
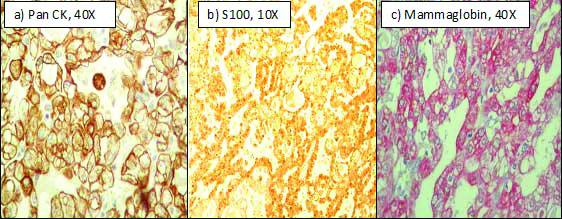
Immunohistochemistry showed strong and diffuse expression of pan CK, EMA, S100, mammaglobin and negative expression of Calponin, GCDFP1, DOG1, ER, PR, Her2neu and SMA [Table/Fig-2]. Based on the above findings, a final diagnosis of Mammary analogue of secretory carcinoma was made. However, confirmatory study of ETV6 gene (12p13) rearrangement was not feasible in our case. Patient is presently disease free seven months after surgery.
Immunohistochemistry :a) Pan CK positive in tumour cells (40X) b) S100 positive (10X) c) Mammaglobin positive (hematoxylin counterstain) (40X).
Discussion
Tumours of salivary gland are rare and have an incidence of 3 per 1,00,000 people [1]. MASC was first described in 2010 by Skálová A et al., as a rare salivary gland tumour with histomorphological and immunohistochemical features similar to secretory carcinoma of the breast [2]. Breast and salivary gland tissues have same ectodermal embryonic origin with an identical ductulo-acinar architecture. Both MASC and SC are composed of microcystic and solid areas with intraluminal abundant vacuolated PAS-positive colloid-like secretions [2].
MASC are diagnosed mainly in the major salivary glands with 70% being in the parotid gland and 7% in in submandibular gland [3]. These tumours have also been found to occur in the minor salivary glands of lip, soft palate, hard palate, base of tongue and buccal mucosa. MASC is most commonly seen in men with a male: female ratio of 1.5:1. The average age of diagnosis is 46 years, with a usual range of 14-77 years [3]. It clinically presents as slow growing, painless mass with few incidentally detected cases and is considered to be a low-grade carcinoma with a relatively favourable prognosis [3].
Our case presented at an age of 17 years as a painless parotid mass without any neural or lymphatic involvement and was provisionally diagnosed as mucoepidermoid carcinoma on FNAC.
MASC can be mistaken for other salivary gland tumours, especially acinic cell carcinoma, low grade mucoepidermoid carcinoma, adenocarcinoma-NOS and salivary duct carcinoma. MASC is well circumscribed unencapsulated, grey-white, brown, or yellow color; with prominent cystic component. Microscopically, MASC is comprised of solid and cystic areas with cysts filled with PAS positive colloid like material and cell with eosinophilic granular or vacuolated cytoplasm and pale nuclei [4]. Strong immunohistochemical positivity for S-100, mammaglobin, and cytokeratin helps distinguish MASC from other salivary gland tumours.
Acinic cell carcinoma is composed of acinar cells which have cytoplasmic PAS-positive, basophilic zymogen-like granules. Bishop JA et al., reported that 19% of MASC had a prior histopathologic diagnosis of mostly ‘zymogen poor’ acinic cell carcinoma. On immunohistochemistry, ACC are negative for S100 protein and mammaglobin and show strong DOG1 staining [5].
Mucoepidermoid carcinoma may mimic MASC with predominantly macrocystic pattern. Mucoepidermoid carcinoma may also express mammaglobin. However, presence of focal squamoid cells, diffuse nuclear p63 expression, and negative S100 protein expression favors diagnosis of mucoepidermoid carcinoma over MASC [4].
Salivary duct carcinoma and adenocarcinoma-NOS can be differentiated from MASC by the lack of bubbly pink cytoplasm which is a constant finding in the latter and by its predominantly intraductal location in the former. Immunohistochemistry is not of much use as both salivary duct carcinoma and adenocarcinoma can also show S100 and mammaglobin expression like MASC. However, the characteristic intraductal proliferation in salivary duct carcinoma can be confirmed by calponin staining [6].
MASC and SC of the breast shows presence of the translocation t(12;15) (p13;q25) forming oncogenic fusion gene ETV6-NTK3, also found in other tumours such as infantile fibrosarcoma, myelogenous leukemia, and congenital mesoblastic nephroma [7]. This fusion forms a chimeric tyrosine kinase causing activation of cell proliferation with increased survival of the tumour cells. Testing for the ETV6-NTRK3 gene fusion is the definitive diagnostic criteria of MASC but such highly specialised testing is not feasible in many laboratories. Therefore, classic histopathology of MASC alongwith strong staining for mammaglobin and S-100, should be sufficient for its diagnosis in the absence of genetic testing [8].
MASC is treated as low-grade carcinoma with a relatively favourable prognosis. It usually does not show neurovascular invasion or local infiltration. However, simple enucleation of the tumour has a higher risk of local recurrence when compared with wide excision. MASCs have a 20% risk of lymph node metastases and 5% risk of distant metastases [9]. Our case was disease free seven months after surgery.
Conclusion
Mammary analogue secretory carcinoma is a rare salivary gland tumour which was often diagnosed as acinic cell carcinoma, mucoepidermoid carcinoma or adenocarcinoma in the past. Histopathology with the aid of appropriate immunohistochemistry can help in the correct diagnosis of this low grade carcinoma and thus help in the appropriate management of MASC.
[1]. Parkin DM, Ferlay J, Curado MP, Bray F, Edwards B, Shin HR, Fifty years of cancer incidence: CI5 I-IXInt J Cancer 2010 127(12):2918-27.10.1002/ijc.2551721351270 [Google Scholar] [CrossRef] [PubMed]
[2]. Skálová A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: ahitherto undescribed salivary gland tumor entityAm J Surg Pathol 2010 34:599-608.10.1097/PAS.0b013e3181d9efcc20410810 [Google Scholar] [CrossRef] [PubMed]
[3]. Sethi R, Kozin E, Remenschneider A, Kozin E, Meier J, VanderLaan P, Mammary analogue secretory carcinoma: update on a new diagnosis of salivary gland malignancyLaryngoscope 2014 124(1):188-95.10.1002/lary.2425423775296 [Google Scholar] [CrossRef] [PubMed]
[4]. Skalova´ A, Mammary analogue secretory carcinoma of salivary gland origin: an update and expanded morphologic and immunohistochemical spectrum of recently described entityHead Neck Pathol 2013 7(suppl 1):S30-36.10.1007/s12105-013-0455-y23821207 [Google Scholar] [CrossRef] [PubMed]
[5]. Bishop JA, Yonescu R, Batista D, Westra WH, Eisele DW, Most nonparotid ‘acinic cell carcinomas’ represent mammary analog secretory carcinomasAm J Surg Pathol 2013 37(7):1053-57.10.1097/PAS.0b013e318284155423681074 [Google Scholar] [CrossRef] [PubMed]
[6]. Stevens TM, Kovalovsky AO, Velosa C, Shi Q, Dai Q, Owen RP, Mammary analog secretory carcinoma, low-grade salivary duct carcinoma, and mimickers: a comparative studyModern Pathology 2015 28:1084-100.10.1038/modpathol.2015.6426089091 [Google Scholar] [CrossRef] [PubMed]
[7]. Knezevich SR, Garnett MJ, Pysher TJ, Beckwith JB, Grundy PE, Sorensen PH, ETV6-NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcomaCancer Res 1998 58:5046-48. [Google Scholar]
[8]. Shuqin W, Yuncheng Li, Guojun Li, Lijun Z, Mammary analogue secretory carcinoma presenting as a salivary gland neoplasm: a first clinical case report of an exceptionally rare tumor in ChinaInt J Clin Exp Med 2016 9(11):22632-37. [Google Scholar]
[9]. Skálová A, Vanecek T, Majewska H, Laco J, Grossmann P, Simpson RH, Mammary analogue secretory carcinoma of salivary glands with high-grade transformation report of 3 caseswith the ETV6-NTRK3 gene fusion and analysis of TP53, beta-catenin, EGFR, and CCND1 genesAm J Surg Pathol 2014 38:23-33.10.1097/PAS.000000000000008824145651 [Google Scholar] [CrossRef] [PubMed]