Introduction
Global status report 2014 of World Health Organization (WHO) for India estimates that Non Communicable Diseases (NCDs) account for 60% of all deaths to which 45% contribution is from Cardiovascular Diseases (CVDs) [1]. Among these CVDs, HF demands more attention in view of rapidly rising prevalence of CVD risk factors and Ischaemic Heart Disease (IHD) especially in young Indians. Increasing life expectancy adds further to the burden of HF [2]. Major risk factors for HF include CAD, hypertension, diabetes, obesity and other heart diseases. WHO estimates suggest a 10% rise of hypertension in a short span of 4 years (2010-2014) [1]. Though there are clear estimates on burden of most other NCDs, no robust data on HF incidence and prevalence in India exists [2]. Conservative estimates reported on the prevalence of HF in India resulting from CAD, hypertension, obesity, diabetes, and Rheumatic Heart Disease (RHD) ranged from 1.3 to 4.6 million, with an annual incidence between 4,91,600 and 1.8 million [3]. A recent INDUS study in rural adults from Northern India, reported HF in 9% cases with prevalence in general community being 1.2/1000 population [4]. Compared to the western world, this is a gross underestimate and suggests paucity of data, rather than actual low prevalence. A study from Northern India in Acute Decompensated HF (ADHF) reported inhospital mortality of 30.8% with 26.3% mortality rate within 6-months post-discharge [5]. Recently published Trivandrum HF registry study, reported that ADHF was the result of IHD (72%) and ‘90 day’ all cause mortality rate of 2.43/1000 person days [6]. Additionally, patients admitted for HF have poorer prognosis since they are often referred late or seek medical advice very late after the onset of HF. The studies indicate lack of robust data rather than heterogeneity. Nevertheless, these numbers raise the alarm for containment of HF-associated morbidity and mortality in India.
However, there are no country-specific guidelines that provide recommendations for effective screening, diagnosis, and management of HF. A consensus opinion from experts in India was recently released [7]. There is a need to update existing consensus recommendations, to match with recent evidence and change, in international guidelines recommendations as published in 2016 European Society of Cardiology (ESC) HF guidelines for the diagnosis and treatment of acute and chronic HF [8]. This 2016 ESC-HF guideline, corroborated new evidence and provided new recommendations for HF management. However, to adapt these guidelines in Indian setting, there is a need for appraisal of the recommendations considering the availability, affordability of the diagnostic and treatment modalities and access to healthcare facilities for patients with HF. Keeping up with the evidence based recommendations, India-specific modifications in the recommendations from ESC guidelines were suggested, by the experts in HF management. This article provides the consensus from a group of experts across the country that agreed upon India-specific recommendations to adopt ESC guidelines for diagnosis and major treatments of chronic HF.
The Expert Panel
The expert panel consisted of cardiologists and physicians (total 16) involved in management of acute and chronic HF. These experts were from different cities like Delhi, West Bengal, Gujarat, Telangana, Bihar; and Tamil Nadu. Each of the experts had over 15 years of experience in treatment of HF. Firstly two authors approached the panellist through mail for participation in the discussion and providing their viewpoints on the guidelines. 2016 ESC-HF guideline was shared with each of the panellist before the meeting. Meeting was coordinated by first two authors who presented the evidence from recent ESC-HF guidelines to the panel. Each recommendation was discussed by the panel members and provided with India specific consensus opinion. Meeting lasted for eight hours.
Approach To The Consensus
The agenda and the purpose of the meeting were known to experts before the meeting. All experts were provided sufficient time to extract the data and streamline the thought process to proceed in meeting. The panel was divided in three parts. In first, two experts presented the ESC guideline recommendations; in second, five experts chaired the session and kept the records of consensus and remaining nine experts contributed to the discussion on each recommendation to arrive at a consensus. Though ESC guidelines provide recommendations on various aspects of HF, panel discussed most practical issues that might be pertinent to Indian setting. The expert consensus opinions are provided in following sections.
Approach to diagnosis
Diagnostic tests for HF: Non invasive and invasive
Pharmacological treatment of HF with reduced ejection fraction
Devise treatment of HF with reduced ejection fraction
Approach To Diagnosis Of HF
ESC guideline defined HF based on 3 criteria into three categories. Presence of symptoms/signs of HF, elevated levels of Natriuretic Peptides (NPs) and one additional criteria (relevant structural heart disease or diastolic dysfunction) were constant but Left Ventricular Ejection Fractions (LVEFs) varied leading to three categories as below [8]:
HF with reduced Ejection Fraction (HFrEF): LVEF <40%
HF with mid range Ejection Fraction (HFmrEF): LVEF 40-49%
HF with preserved Ejection Fraction (HFpEF): LVEF ≥50%
However, the panel suggested only two categories of HFrEF and HFpEF based on LVEF of <45% or ≥45% for Indian setting. It was further suggested that HFmrEF can be considered for research purpose at that moment.
ESC 2016 guidelines recommended a stepwise algorithm to diagnose HF in non acute setting [8]. Classical clinical symptoms and signs with any Electrocardiogram (ECG) abnormality and evaluation by Echocardiography (ECHO), confirm the diagnosis of HF. In absence of all classical clinical symptoms and signs or ECG abnormalities, HF is unlikely. When available, NP should be determined. Level of N-terminal -pro brain NP (Nt-ProBNP) ≥125 pg/mL or BNP ≥ 35 pg/mL suggests HF. With such elevated NPs levels, confirmation by ECHO is necessary. If NPs are not elevated, or ECHO is normal, HF is unlikely. [Table/Fig-1] describes the approach to diagnosis of chronic HF.
Approach to diagnosis of HF.
a) Includes previous history of CAD, hypertension, use of diuretics, exposure to toxins, orthopnoea, paroxysmal nocturnal dyspnoea; b) Physical findings like rales, ankle oedema in both legs, third heart sound or murmur, raised JVP, lateral displacement of or broadened-apical beat.
NP: Natriuretic Peptides, BNP: Brain Natriuretic Peptides
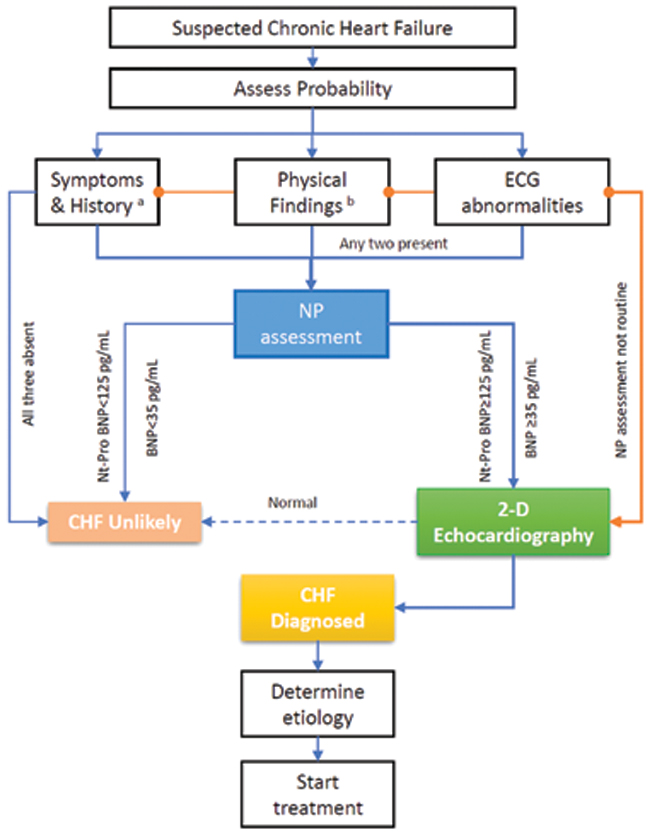
The panel advised that in areas where biomarkers are not available, diagnosis is based on clinical presentation and ECHO if available. The panel finds it useful to perform both NP and ECHO investigation whenever available. Role of BNP and/or Nt-ProBNP is to exclude the diagnosis of HF and will increase the certainty when combined with ECHO. A systematic review from Hill SA et al., finds that both biomarkers perform well to rule-out and less well to rule-in the HF diagnosis [9]. In Indian context, panel identified that cost-benefit ratio of investigations need to be considered. However, evidence suggests that using these biomarkers as screening test can reduce use of ECHO in substantial number of patients for diagnosis. Ferrandis MJ et al., reported €2-5 million per year per million inhabitant reduction in cost with use of these biomarkers [10]. At peripheral levels, ECG should be done in all suspected cases. A completely normal ECG might rule-out HF. Although, there are no specific ECG features of HF, atrial and ventricular arrhythmias, may be noted commonly. Presence of Atrial Fibrillation (AF), ventricular arrhythmia and Left Bundle Branch Block (LBBB) represent a worse prognosis in HF [11]. If doubt persisted about the diagnosis, a referral to higher centre should be done.
Expert opinion: In addition to classical clinical presentation and ECG, evaluation with Nt-ProBNP or BNP and ECHO should be done, whenever available, to diagnose HF.
Diagnostic Tests For HF: Non Invasive
Baseline routine investigations: In routine, ESC guidelines recommend following tests to determine the suitability of treatments, to identify any reversible or treatable causes of HF and co-morbidities if any and these include [8]: Haemoglobin, white cell count, serum biochemistry-sodium, urea, creatinine, liver function tests, blood glucose, glycosylated haemoglobin, lipid profile, TSH, serum ferritin, transferrin saturation and natriuretic peptides. Additional tests - pertaining to specific aetiologies of HF, can be undertaken in suspicious cases (12-lead ECG).
Expert panel agreed that these tests are essential in guiding the diagnosis and treatment of HF. Panel suggested lipid profile assessment in routine, may not be necessary in HF cases except for CAD associated HF. An observational study has reported inverse relationship of lipid profile with Right Ventricle End Diastolic Diameter (RVEDD) and remained unchanged, even after adjustment, for patients characteristics like age, gender, smoking status, physical activity levels, comorbidities, and medication use [12].
Expert opinion: These baseline investigations are mandatory in all HF cases. Lipid profile may be or may not be included taking in to consideration, the aetiology of HF.
ST2 was one of the marker, considered during discussion on diagnosis of HF. However, ST2 was regarded more of prognostic than diagnostic marker [13,14]. At this moment, ST2 is not being routinely utilised in clinical setting in India. Further evidence from India will be necessary to use it as a routine prognostic marker.
Expert opinion: ST2 is not advised as a diagnostic marker and more evidence is necessary to advise its inclusion, in routine HF management, as a prognostic marker.
Serum iron studies were identified as one of the important evaluations as most patients of HF develop iron deficiency [15,16]. Panel suggested that nearly 50-70% patients with HF may have iron deficiency. A study from Cohen-Solal A et al., [17] in HF patients observed prevalence of anaemia to be 68% in males and 52% in females (haemoglobin <13 gm/dL and <12 gm/dL respectively). Even in non anaemic patients, iron deficiency was present in 57% men and 79% women. Assessing iron profile after 24 hours of hospitalisation should be taken as a routine practice especially in Indian setting since the nutritional iron deficiency is significantly prevalent [18].
Expert opinion: Serum iron study especially serum ferritin and transferrin saturation, are to be evaluated in all HF cases, to rule out iron deficiency and need for treatment with intravenous iron.
Jugular Venous Pressure (JVP) being a readily identifiable factor, panel discussed its importance for diagnosis of HF. It is a part of clinical judgement. Interesting observation from Breathing Not Properly (BNP) study [19] suggest that alone clinical judgment had 49% sensitivity when 80% certainty level was considered. Addition of BNP (100 pg/mL) to clinical judgement was reported to increase the diagnostic accuracy from 74% to 81%. Thus, in acute setting, BNP was suggested as point of care assessment in addition to clinical judgment.
Expert opinion: JVP alone cannot establish a diagnosis of HF. It can supplement clinical and ECHO examination.
Exercise testing: ESC guidelines recommend exercise testing as evaluation for patients undergoing heart transplantation and/or mechanical circulatory support. Further, it can be considered to optimise prescription of exercise testing, to assess the dyspnoea of indeterminate cause and may be considered to identify reversible myocardial ischaemia [8]. Panel had differences of opinion on suggesting exercise testing in HF. Some argued that it is necessary to perform an exercise testing before a coronary angiogram to determine the myocardial ischemia level, whereas others suggested to not to use it, to assess the viability. Exercise testing was advised only for assessing filling pressure in HF.
Expert opinion: Need for expertise in evaluating patients using cardiopulmonary exercise testing may limit its clinical use in Indian setting. In centers where it is used and employed in patient management, it can be performed under controlled conditions before a coronary angiogram, to assess myocardial viability.
Cardiac Imaging
Chest X-ray: It was recommended to detect or exclude alternative pulmonary and other diseases resulting in dyspnoea. In acute setting, chest X-ray may also be used to detect pulmonary congestion and/or oedema in HF. Panel agreed to this recommendation from ESC guidelines and suggested routine use of X-ray at the time of presentation and in follow-up as necessary in all cases of HF.
Expert opinion: Chest X-ray is essential in all HF cases to understand associated respiratory pathologies.
Transthoracic Echocardiography (TTE): ESC guidelines recommend transthoracic echocardiography (TTE) for conditions as summarised below:
To assess myocardial structure and function in suspected HF and to establish diagnosis of HF with reduced, mid-range or preserved Ejection Fraction (EF)
To assess EF to identify suitable patients for treatment with pharmacological and device based therapies in HF with reduced EF (HFrEF)
To assess valve disease, Right Ventricle (RV) function, pulmonary artery pressure in established HF cases, to identify patients suitable for correction of valve disease
To assess the myocardial structure and function in patients who have been exposed to potentially cardiotoxic medication e.g., chemotherapeutic agents
Additionally, tissue Doppler velocities and deformation indices to be considered in TTE in patients who are at risk of HF to assess myocardial dysfunction at preclinical stage [8]
Expert opinion: Unanimous agreement of the panel on these recommendations for Indian setting as well.
Cardiac Magnetic Resonance (CMR): CMR imaging is recommended in ESC guidelines in following situations [8]:
To assess myocardial structure and function in patients with poor acoustic window and patients with complex congenital heart disease
With late gadolinium enhancement, in patients with Dilated Cardiomyopathy (DCM) to distinguish ischaemic and non ischaemic damage in cases of equivocal clinical or other imaging
For catheterisation of myocardium in suspected myocarditis, amyloidosis, sarcoidosis, Chaga’s disease, Fabry’s disease, non-compaction cardiomyopathy, and hemochromatosis
Non invasive stress testing ma-y be considered to assess myocardial ischaemia and viability in HF with CAD before deciding on revascularisation
The panel agreed to these recommendations. As IHD is the most common aetiology of HF, emphasis was stressed on anatomical disease. In HF without symptoms, suspected anatomical lesion in coronaries necessitates coronary angiogram to understand the anatomical disease. If available, contrast ECHO or CMR testing may prove beneficial. Cardiac MRI assisted quantitation of left and right ventricular volumes and the systolic function are important determinants of prognosis and aid in clinical decision making in management of HF. Further, CMR with Delayed Gadolinium Enhancement (DGE) can differentiate ischaemic and non ischaemic cardiomyopathy in HF [20]. CAD being one of the most prevalent CVD in India, [21] exclusion of CAD as an aetiology of HF demands use of investigations like coronary angiography, cardiac MRI and ECHO.
Expert opinion: CMR is advised to know the aetiology of HF including cardiomyopathies and to understand the cardiac viability (with use of pharmacological stress test) in selected cases where cost concerns are not raised.
Taking into consideration the availability, costs, and expertise of reporting on CMR in India, ECHO was advised as the principal imaging test for HF with CMR in selected cases. Routinely, EF reported in ECHO examination is being performed in standard M-mode which was disagreed by panel. M-mode echocardiography should not be used for the left ventricle. The Panel suggested modified biplane Simpson method to assess EF and provide data on global longitudinal strain. The Panel pointed that in addition to EF, reporting on Global Longitudinal Strain (GLS) in every individual with HF may provide more information about the disease than EF alone. The prognostic value of GLS has been proven in various clinical studies [22,23]. Indian Academy of Echocardiography (IAE) advises Simpson’s method to obtain ventricular volumes and EF and provides measurement of longitudinal strain as optional [24]. Panel urged echocardiography analysts to understand and adopt the standards advised by IAE.
Expert opinion:
Use biplane Simpson method to report on EF using ECHO.
Reporting on GLS is necessary in addition to EF in management of HF.
IAE standards and practices can be adopted by Indian ECHO analysts to help with better ECHO examination and reporting.
Thoracic ultrasound: ESC recommended thoracic ultrasound to confirm pulmonary congestion and pleural effusion in acute HF can be considered. The panel agreed this to be performed in selected cases where predominant respiratory symptoms are present or chest X-ray provides inconclusive findings.
Inferior Vena Cava Diameter (IVC-D): IVC-D may be assessed to know the volume status of HF patients [8]. Panel agreed to these recommendations for use in Indian settings. Assessing IVC-D is important to determine the treatment strategy. When required in any patient, panel suggested to perform its assessment at baseline and at discharge, to determine the prognosis. Patients with fluid overload condition like cardio-renal syndrome, a dilated IVC-D dictates continuation of diuretics. In advanced decompensated HF, Lee HF et al., reported a significant prediction of adverse outcomes in patients with dilated IVC (>21 mm) [25]. They also reported dilated IVC as predictor of worsening renal function.
Expert opinion: Ultrasound examination of thorax to know signs of congestion in acute HF is advised in selected cases. Assessment of IVC diameter using ultrasound to understand fluid dynamics may be done prognostically in management of HF at time of hospitalisation, during and before discharge.
Diagnostic Tests for HF: Invasive
Coronary angiography: ESC guidelines recommend coronary angiography in HF with high probability of CAD and in presence of ischaemia as identified by non invasive investigations, to establish the diagnosis of CAD and its severity [8].
Cardiac CT: Cardiac CT may be done in HF with low probability of CAD or in patients with equivocal non invasive stress test to rule-out coronary artery stenosis. Reassessment of myocardial structure and function using non invasive imaging is recommended in following situations:
In worsening HF symptoms including ADHF or any other important CV event
In HF treated with evidence-based pharmacotherapy in maximal tolerated doses before decision on devise implantation
In patients exposed to myocardial damaging agents e.g., chemotherapy where serial assessments may be undertaken [8]
Expert opinion: We agree to these recommendations. Coronary angiography can be the first-line investigation in HF evaluation as it is widely available, not too costly, and often other non invasive tests remain inconclusive. Stress tests have low yield in patients with HF. Cardiac CT is not advised for diagnosis as the instillation of dye may increase the volume load especially in moderate to severe HF and development of tachycardia may be problematic. A perfusion scan after coronary angiography is useful in majority of patients.
Endomyocardial Biopsy (EMB): ESC guidelines recommend EMB in rapidly progressive HF despite standard medical treatment to identify specific aetiology of HF that can be confirmed only after biopsy and for such condition effective treatments are available [8]. The panel agreed to these recommendations and advised to restrict EMB to specific situations where there is strong suspicion of aetiology that needs confirmation with biopsy only. However, panel pointed the need for expertise to perform myocardial biopsy and suggested it should be restricted only in expert hands to avoid complications and yield the better results. A study by Talwar KK et al., from India reported diagnostic yield of 15.4% with EMB in dilated cardiomyopathy [26].
Expert opinion: EMB is advised only in selected cases where all other assessment fails to show any specific cause and where structural heart disease is suspected. It should be performed in expert hands where aetiology of HF is unclear.
Right heart catheterisation: Right heart catheterisation using a pulmonary artery catheter in severe HF is recommended by ESC to evaluate heart transplantation and mechanical circulatory support. It is also considered to confirm pulmonary hypertension in patients who had suspicion on ECHO and to determine its reversibility when correction of structural or valvular heart disease is planned [8]. The panel agreed to these recommendations.
Guidelines further advise right heart catheterisation to modify HF therapy in patients who remain severely symptomatic despite standard therapy and have unclear haemodynamic status [8]. Though not routinely done, right heart catheterisation can be an additional helpful test in HF with grey areas where diagnosis is difficult especially in diastolic HF. However, routine use was not advised from the panel.
Expert opinion: Right heart catheterisation is not advised routinely but it can be helpful in HF diagnosis where other modalities are unable to provide specific diagnosis.
Treatment of HF with Reduced EF
Pharmacological treatment: In patients with symptomatic HFrEF, in all cases diuretics are recommended to relieve the symptoms and signs of congestion. ESC guidelines recommend therapy initiation with Angiotensin Converting Enzyme Inhibitors (ACEIs) and Beta Blocker (BB). If non symptomatic and LVEF above 35%, continue the same treatment. In patients who remain symptomatic and have LVEF 35% or lower, addition of Mineralocorticoid Receptor Antagonist (MRA) is recommended. Monitor symptoms of HF to further strategy. If asymptomatic, continue the three-drug treatment. If patient remain symptomatic, despite maximal tolerated doses of ACEIs, BB and MRA, following actions are recommended:
ACEIs (or angiotensin receptor blocker) tolerated - Replace with Angiotensin-Neprilysin Receptor Inhibitor (ARNI)
In sinus rhythm, QRS duration ≥130 milliseconds - evaluate need for cardiac resynchronisation
Sinus rhythm, heart rate 70 and above beats per minute - Ivabradine
These treatments may be combined as needed. Despite this if patient remains symptomatic, addition of digoxin, hydralazine/isosorbide dinitrate, LVAD or heart transplantation may be considered.
In patients who have LVEF 35% or below, despite optimal medical therapy, or have history of symptomatic ventricular tachycardia or fibrillation, Implantable Cardioverter-Defibrillator (ICD) implantation is advised.
The panel advised MRA in all cases of HF even in mild HF irrespective of other therapies unless contraindicated. Panel referred to Eplerenone in Mild Patients Hospitalisation and Survival Study in Heart Failure (EMPHASIS-HF) trial [27] which showed that compared to placebo, eplerenone reduced risk of death and hospitalisation in patients with systolic HF and mild symptoms. Subgroup analysis of EMPHASIS-HF trial has proven eplerenone’s role in preventing re-hospitalisation when initiated soon after discharge in HF with mild symptoms [28]. In patients with recent ST Elevation Myocardial Infarction (STEMI) without HF, eplerenone when added to acute therapy of STEMI significantly reduced primary composite endpoint of CV mortality, re-hospitalisation, or extended initial hospital stay, due to diagnosis of HF, sustained ventricular tachycardia or fibrillation, ejection fraction ≤40%, or elevated BNP/Nt-ProBNP (18.2% in eplerenone vs. 29.4% in placebo treatment, Hazard Ratio (HR): 95% Confidence Interval (CI), 0.45-0.76; p<0.0001) and this was primarily driven by lowering of BNP/Nt-ProBNP levels [29]. This evidence clearly suggests that eplerenone has potential even to prevent HF in asymptomatic patients. This evidence identified by panel to advice eplerenone even in patients with mild HF or asymptomatic HF. However, eplerenone is said to be an underused medication in the setting of HF [30] which needs to be re-emphasised. Monitoring of potassium and creatinine is advised. [Table/Fig-2] depicts approach to treatment of HF with reduced ejection fraction.
Treatment approach to HF with reduced EF.
a) Achieve optimal tolerated doses of these medications before adding further treatments;
b) Additional treatments include digoxin, hydralazine, isosorbide dinitrate, left ventricular assist device, heart transplantation
ACEI: Angiotensin Converting Enzyme Inhibitor, BB: Beta Blocker, MRA: Mineralocorticoid Receptor Antagonist, LVEF: Left Ventricular Ejection Fraction, ARB: Angiotensin Receptor Blocker, ARNI: Angiotensin Receptor Neprilysin Inhibitor, HR: Heart Rate, CRT: Cardiac Resynchronization Therapy, OMT: Optimal Medical Therapy, VT/VF: Ventricular Tachycardia or Fibrillation, ICD: Implantable Cardioverter Defibrillator
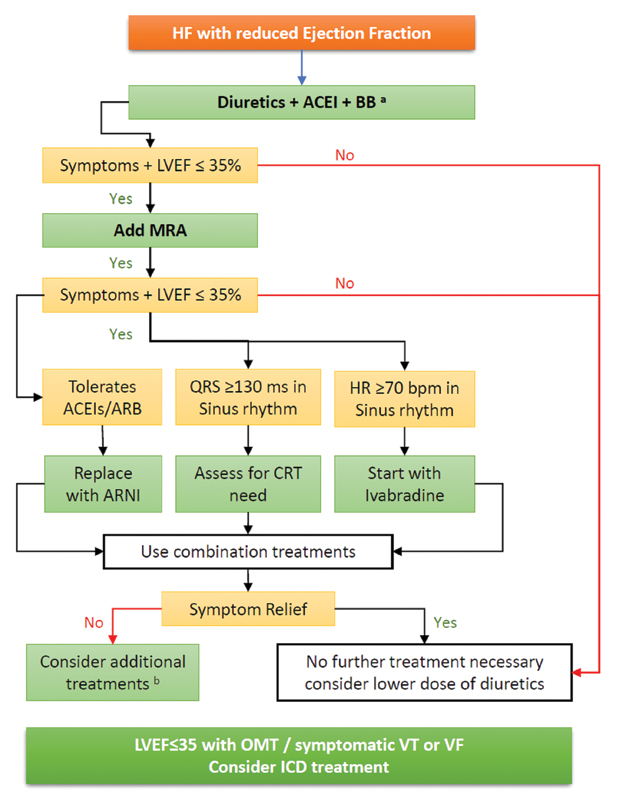
Expert opinion: Even in asymptomatic patients with LVEF 35% or below, addition of MRA should be considered to ACEIs and BB therapy.
In patients with diabetes mellitus, MRA may be used in patients with LVEF < 40%
The panel advised that for use of ARNI, availability and cost are two major concerns. Concerns regarding angio-oedema were raised in Angiotensin-Converting- Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial [31]. However, panel identified that angio-oedema with ARNI was not seen frequently in PARADIGM-HF trial but watchfulness for its occurrence was advised.
With regards to evaluating need for CRT, panel considered Left Bundle Branch Block (LBBB) morphology being more essential than QRS duration only. In Right Bundle Branch Block (RBBB), CRT is contraindicated.
Panel agreed on recommendation of ivabradine in cases of HF with HR above 70 bpm in sinus rhythm. The panel identified that in Indian patients, HR is usually raised in HF despite receiving BB. Wherever possible, heart rate assessment may be performed by a baseline ECG or portable inexpensive pulse oximetry devices for accurate documentation.
Expert opinion: In patients who remained symptomatic despite diuretic, ACEIs, BB and MRA, replacing ACEIs with ARNI should be considered taking in to account the availability, affordability, and patient preferences. LBBB morphology is more essential than QRS duration only to determine the CRT applicability in such patients. Accurate HR measurement to be assured in Indian HF patients for assessing the use of ivabradine in HF.
The panel agreed to following pharmacotherapy recommendations from ESC guidelines [8]. In patients of HFrEF, to reduce risk of HF hospitalisations and death:
ACEI is recommended in addition to BB in symptomatic cases
BB is recommended in addition to ACEI for stable, symptomatic patients
MRA is recommended for those who remain symptomatic despite treatment with ACEI and BB.
Here, panel advised MRA use in all HF cases, even in asymptomatic ones with monitoring of potassium
Diuretics to improve symptoms and exercise capacity in patients having clinical signs and symptoms of congestion and should be considered
ARNI is recommended to replace ACEI to further reduce risk of HF hospitalisations and death who remain symptomatic despite ACEIs, BB and MRA
Ivabradine in symptomatic patients having LVEF 35% or below in sinus rhythm with HR 70 or more bpm despite evidence-based dose of BB (or even in those who are unable to tolerate BB), ACEI and MRA treatment.
ARB is recommended in symptomatic patients who are unable to tolerate ACEIs (continue BB and MRA) or even in those cases who remain symptomatic even with BB treatment and are unable to tolerate MRA
Hydralazine and isosorbide dinitrate to be considered in black patients with LVEF ≤ 35% or with LVEF ≤45% combined with dilated LV in patients with NYHA Class III-IV dyspnoea despite treatment with ACEI, BB and MRA or may be used in patients who are unable to tolerate ACEI or ARB (or contraindicated)
Digoxin may be considered in symptomatic patients in sinus rhythm despite ACEI (or ARB), BB and MRA.
With digoxin, panel pointed that measuring serum levels of digoxin is essential to keep it between 0.5-0.8 ng/mL as these serum levels showed lower mortality compared to those with levels >1 ng/mL [32].
In HF with AF, panel pointed that it is necessary to be careful while using digoxin as the chances of using higher dose to control the rate lead to more harm than benefit patients. The Retrospective Evaluation and Assessment of Therapies in AF (TREAT-AF) study [33] in newly diagnosed AF reported greater mortality with digoxin even after multivariate adjustment (HR: 1.26, 95%CI: 1.23-1.29, p<0.001). Thus, panel advised caution while using digoxin for rate control in HF with AF.
ESC guidelines recommend that following treatment can cause harm in symptomatic HFrEF [8]: Thiazolidinediones, Non Steroidal Anti-Inflammatory Drugs (NSAIDs) or Cyclooxygenase-2 (COX-2) inhibitors, Diltiazem or verapamil, ARB in combination with ACEIs and MRA (triple therapy should be avoided at all cost).
Device Treat reatment of HF With Reduced EF
Implantable Cardioverter Defibrillator
In secondary prevention: ESC guideline recommends ICD to lower risk of sudden death and all-cause death in patients who recovered from arrhythmia causing haemodynamic instability and patients who have expected survival of over one year with good functional status [8]. Panel agreed to this recommendation.
In primary prevention: ICD is recommended in symptomatic HF (NYHA II-III) with LVEF ≤35% despite ≥3 months of optimal medical treatment in those who are expected to live over one year with good functional status and have.
IHD
DCM
Here panel pointed that, recent Danish study to Assess the efficacy of ICDs in patients with Non Ischaemic Systolic Heart Failure on Mortality (DANISH) trial [34] found no difference in all-cause mortality in non ischaemic HF patients treated with prophylactic ICD + usual care (21.6%) against usual care alone (23.4%) (HR 0.87; 95% CI, 0.68 to 1.12; p=0.28). Panel also pointed that CRT can also reduce factor in such cases but CRT use was equalled in both groups (58%) of DANISH trial [34]. However, panel suggested that ICD implantation for primary prevention of SCD in ischaemic systolic HF is unequivocally accepted; however, same in cases of non ischaemic systolic HF should be guarded and guided by age, risk for non sudden death and comorbidities.
Panel further advised that prescribing ICD to individuals with LVEF ≤35% despite medical treatment will increase the number of patients eligible for ICD in Indian setting and that can be enormous economic burden in healthcare. Cost associated with ICD is a major challenge for their effective implementation [35]. From India, a recent multicentric survey on use of ICDs identified that in ICD implantation 65% were single chamber, CAD was the aetiology in 58.5% cases, patients with LVEF ≤35% accounted for 88% of study and indications were nearly equal for primary and secondary prevention [36]. As the LVEF alone has limitations for predicting the mortality outcomes, ICD implantation need to consider factors besides LVEF like NYHA class, CAD load, QRS width, LBBB, heart rate variability and others [37]. Beside these clinical and electrophysiological parameters, the panel suggested consideration of disability and quality of life for deciding on use of ICD. As urgent need of India specific guidelines on use of ICD was warranted by the panel.
ESC guidelines state that ICD is not recommended in following conditions [8]:
Within 40 days of an MI
In NYHA Class IV patients with severe symptoms, that are not responding to pharmacological treatment unless these patients are candidate for CRT, ventricular assist device, or cardiac transplantation
Expert opinion:
ICD should only be restricted to ischaemic aetiology HF.
Besides LVEF≤35%, consider clinical, electrophysiological parameters, quality of life and cost, before deciding on prophylactic ICD implantation.
Treatment of HF with Preserved and Mid-range Ejection Fraction
ESC 2016 guidelines recommend to actively screen for presence of any cardiovascular and non cardiovascular comorbidities in patients of HFpEF and HFmrEF. If present, these should be managed with safe and effective interventions. If there are symptoms of congestion in either category of HF, diuretics are recommended to provide symptomatic relief [8]. Panel members agreed to these recommendations. However, as suggested earlier, panel considered use of HFpEF or HFmrEF for research purpose.
Expert opinion: Treatment of comorbidities is essential in HFpEF or HFmrEF. Diuretics should be considered for relief of congestive symptoms, if any. At present, use of such classification can be considered for research purpose.
Summary
BNP/Nt-ProBNP assessment is essential in addition to ECHO examination and are complementary in diagnosis of HF. Routine laboratory investigations, ECG and chest X-ray are necessary in all HF cases. A completely normal ECG may suggest a diagnosis other than HF. Iron studies especially serum ferritin and transferrin saturation are necessary to rule out iron deficiency and to decide IV iron therapy. ECHO is the most primary investigation to diagnose HF. Reporting of EF with biplane Simpson method along with reporting of global longitudinal strain to determine prognosis should be routine practice. Exercise testing should be reserved at centers with expertise available to perform it. CMR imaging is to be done in selected cases where expertise available to assess the viability and functionality of myocardium before subjecting to coronary angiography. Coronary angiography can be first-line investigation to determine the coronary anatomy in HF due to CAD.
Besides diuretic, ACEIs, and BB, MRA is advised in all HF cases including the asymptomatic ones with monitoring of potassium. Replacing ACEI/ARB with ARNI in selected cases should be considered after taking in to account, affordability and availability to derive further mortality benefits. In Indian HF cases, HR below 70 bpm is rarely achieved even in patients receiving BB treatment paving way for use of ivabradine. Digoxin is indicated only in severe symptomatic cases who fail to respond to all previous treatments and if patients developed AF. Serum levels between 0.5-0.8 ng/mL are advised. ICD is advised in ischemic HF cases after taking into consideration LVEF, clinical, electrophysiological parameters, cost and quality of life of patient.
Author’s Contribution
Authors would like to recognize the contribution of the esteemed panellist of this consensus document in providing their valuable insights for reaching the consensus: Dr. RK Agrawal, Dr. Sandeep Bansal, Dr. MK Das, Dr. Jyoti Deb, Dr. Anjan Lal Dutta, Dr. Peeyush Jain, Dr. Tiny Nair, Dr. Raja Ray, Dr. KK Sinha, Dr. Sivakadaksham Natarajan, Dr. Saumitra Ray, Dr. JP Sawhney.
[1]. Global status report on noncommunicable diseases 2014, World Health Organiation. 2014. Available from http://www.who.int/nmh/publications/ncd-status-report-2014/en/ Accessed on 22 May 2017 [Google Scholar]
[2]. Prabhakaran D, Jeemon P, Roy A, Cardiovascular diseases in India: current epidemiology and future directionsCirculation 2016 133(16):1605-20.10.1161/CIRCULATIONAHA.114.00872927142605 [Google Scholar] [CrossRef] [PubMed]
[3]. Huffman MD, Prabhakaran D, Heart failure: epidemiology and prevention in IndiaNational Medical Journal of India 2010 23(5):283-88. [Google Scholar]
[4]. Chaturvedi V, Parakh N, Seth S, Bhargava B, Ramakrishnan S, Roy A, Heart failure in India: The INDUS (INDia Ukieri Study) studyJournal of the Practice of the Cardiovascular Sciences 2016 2(1):28-35.10.4103/2395-5414.182988 [Google Scholar] [CrossRef]
[5]. Seth S, Khanal S, Ramakrishnan S, Gupta N, Bahl V, Epidemiology of acute decompensated heart failure in India : The AFAR study (Acute failure registry study)Journal of the Practice of the Cardiovascular Sciences 2015 1(1):35-38.10.4103/2395-5414.157563 [Google Scholar] [CrossRef]
[6]. Sivadasanpillai H, Ganapathy S, Thajudeen A, Sunitha V, Govindan V, Charantharayil BG, Clinical presentation, management, and in-hospital outcomes of patients admitted with decompensated heart failure in Trivandrum, Kerala, India: the Trivandrum heart failure registryEuropean Journal of Heart Failure 2015 17(8):794-800.10.1002/ejhf.28326011246 [Google Scholar] [CrossRef] [PubMed]
[7]. Seth S, Bhargava B, Maulik SK, McDonagh A, Saxena T, Airan B, Consensus statement on management of chronic heart failure in IndiaJournal of the Practice of the Cardiovascular Sciences 2015 1(2):105-12.10.4103/2395-5414.166340 [Google Scholar] [CrossRef]
[8]. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESCEuropean Journal of Heart Failure 2016 37(27):2129-200. [Google Scholar]
[9]. Hill SA, Booth RA, Santaguida PL, Don-Wauchope A, Brown JA, Oremus M, Use of BNP and NT-proBNP for the diagnosis of heart failure in the emergency department: A systematic review of the evidenceHeart Failure Reviews 2014 19(4):421-38.10.1007/s10741-014-9447-624957908 [Google Scholar] [CrossRef] [PubMed]
[10]. Ferrandis MJ, Ryden I, Lindahl TL, Larsson A, Ruling out cardiac failure: cost-benefit analysis of a sequential testing strategy with NT-proBNP before echocardiographyUpsala Journal of Medical Sciences 2013 118(2):75-79.10.3109/03009734.2012.75147123230860 [Google Scholar] [CrossRef] [PubMed]
[11]. Shamsham F, Mitchell J, Essentials of the diagnosis of heart failureAmerican Family Physician 2000 61(5):1319-28. [Google Scholar]
[12]. Chen Y, He XM, Meng H, Zhao QZ, Zhen YZ, Tian L, Relationship between lipids levels and right ventricular volume overload in congestive heart failureJournal of Geriatric Cardiology 2014 11(3):192-99. [Google Scholar]
[13]. Bhardwaj A, Januzzi JL, ST2: a novel biomarker for heart failureExpert Review of Molecular Diagnostics 2010 10(4):459-64.10.1586/erm.10.2520465500 [Google Scholar] [CrossRef] [PubMed]
[14]. Villacorta H, Maisel AS, Soluble ST2 testing: a promising biomarker in the management of heart failureArquivos Brasileiros de Cardiologia 2016 106(2):145-52.10.5935/abc.2015015126761075 [Google Scholar] [CrossRef] [PubMed]
[15]. Jankowska EA, Von Haehling S, Anker SD, MacDougall IC, Ponikowski P, Iron deficiency and heart failure: Diagnostic dilemmas and therapeutic perspectivesEuropean Heart Journal 2013 34(11):816-26.10.1093/eurheartj/ehs22423100285 [Google Scholar] [CrossRef] [PubMed]
[16]. Fitzsimons S, Doughty RN, Iron deficiency in patients with heart failureEuropean Heart Journal-Cardiovascular Pharmacotherapy 2015 1(1):58-64.10.1093/ehjcvp/pvu01627533968 [Google Scholar] [CrossRef] [PubMed]
[17]. Cohen-Solal A, Damy T, Terbah M, Kerebel S, Baguet JP, Hanon O, High prevalence of iron deficiency in patients with acute decompensated heart failureEuropean Journal of Heart Failure 2014 16(9):984-91.10.1002/ejhf.13925065368 [Google Scholar] [CrossRef] [PubMed]
[18]. Anand T, Rahi M, Sharma P, Ingle GK, Issues in prevention of iron deficiency anemia in IndiaNutrition 2014 30(7):764-70.10.1016/j.nut.2013.11.02224984990 [Google Scholar] [CrossRef] [PubMed]
[19]. McCullough PA, Nowak RM, McCord J, Hollander JE, Herrmann HC, Steg PG, B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) multinational studyCirculation 2002 106(4):416-22.10.1161/01.CIR.0000025242.79963.4C12135939 [Google Scholar] [CrossRef] [PubMed]
[20]. Muzzarelli S, Ordovas K, Higgins CB, Cardiovascular MRI for the assessment of heart failure: Focus on clinical management and prognosisournal of Magnetic Resonance Imaging 2011 11(2):275-86.10.1002/jmri.2243321274968 [Google Scholar] [CrossRef] [PubMed]
[21]. Gupta R, Mohan I, Narula J, Trends in coronary heart disease epidemiology in IndiaAnnals of Global Health 2016 82(2):307-15.10.1016/j.aogh.2016.04.00227372534 [Google Scholar] [CrossRef] [PubMed]
[22]. Krishnasamy R, Isbel NM, Hawley CM, Pascoe EM, Burrage M, Leano R, Left ventricular global longitudinal strain (GLS) is a superior predictor of all-cause and cardiovascular mortality when compared to ejection fraction in advanced Chronic Kidney DiseasePLoS One 2015 10(5):e012704410.1371/journal.pone.012704425978372 [Google Scholar] [CrossRef] [PubMed]
[23]. Dahou A, Bartko PE, Capoulade R, Clavel MA, Mundigler G, Grondin SL, Usefulness of global left ventricular longitudinal strain for risk stratification in low ejection fraction, low-gradient aortic stenosis: results from the multicenter True or Pseudo-Severe Aortic Stenosis studyCirculation: Cardiovasc Imaging 2015 8(3):e00211710.1161/CIRCIMAGING.114.00211725681417 [Google Scholar] [CrossRef] [PubMed]
[24]. Burkule N, Bansal M, Mehrotra R, Venkateshvaran A, Indian academy of echocardiography performance standards and recommendations for a comprehensive transthoracic echocardiographic study in adultsJournal of the Indian Academy of Echocardiography & Cardiovascular Imaging 2017 1:01-17. [Google Scholar]
[25]. Lee HF, Hsu LA, Chang CJ, Chan YH, Wang CL, Ho WJ, Prognostic significance of dilated inferior vena cava in advanced decompensated heart failureThe International Journal of Cardiovascular Imaging 2014 30(7):1289-95.10.1007/s10554-014-0468-y24939288 [Google Scholar] [CrossRef] [PubMed]
[26]. Talwar KK, Varma S, Chopra P, Wasir HS, Endomyocardial biopsy-technical aspects experience and current status: An Indian perspectiveInternational Journal of Cardiology 1994 43(3):327-34.10.1016/0167-5273(94)90215-1 [Google Scholar] [CrossRef]
[27]. Zannad F, McMurray JJV, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Eplerenone in patients with systolic heart failure and mild symptomsNew England Journal of Medicine 2011 364(1):11-21.10.1056/NEJMoa100949221073363 [Google Scholar] [CrossRef] [PubMed]
[28]. Girerd N, Collier T, Pocock S, Krum H, Mcmurray JJ, Swedberg K, Clinical benefits of eplerenone in patients with systolic heart failure and mild symptoms when initiated shortly after hospital discharge : analysis from the EMPHASIS-HF trialEuropean Heart Journal 2015 36(34):2310-17.10.1093/eurheartj/ehv27326093641 [Google Scholar] [CrossRef] [PubMed]
[29]. Montalescot G, Pitt B, Lopez E, Hamm CW, Flather M, Verheugt F, Early eplerenone treatment in patients with acute ST-elevation myocardial infarction without heart failure: the randomized double-blind reminder studyEuropean Heart Journal 2014 35(34):2295-302.10.1093/eurheartj/ehu16424780614 [Google Scholar] [CrossRef] [PubMed]
[30]. Abuannadi M, O’Keefe JH, Eplerenone: an underused medication?Journal of Cardiovascular Pharmacological Therapeutics 2010 15(4):318-25.10.1177/107424841037194620876342 [Google Scholar] [CrossRef] [PubMed]
[31]. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Angiotensin-neprilysin inhibition versus enalapril in heart failureNew England Journal of Medicine 2014 371(11):993-1004.10.1056/NEJMoa140907725176015 [Google Scholar] [CrossRef] [PubMed]
[32]. Morris SA, Hatcher HF, Reddy DK, Digoxin therapy for heart failure: an updatAmercan Family Physician 2006 74(4):613-18. [Google Scholar]
[33]. Turakhia MP, Santangeli P, Winkelmayer WC, Xu X, Ullal AJ, Than CT, Increased mortality associated with digoxin in contemporary patients with atrial fibrillation: findings from the TREAT-AF studyJournal of the American College of Cardiology 2014 64(7):660-68.10.1016/j.jacc.2014.03.06025125296 [Google Scholar] [CrossRef] [PubMed]
[34]. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, Defibrillator implantation in patients with nonischemic systolic heart failureNew England Journal of Medicine 2016 375(13):1221-30.10.1056/NEJMoa160802927571011 [Google Scholar] [CrossRef] [PubMed]
[35]. Boriani G, Cimaglia P, Biffi M, Martignani C, Ziacchi M, Valzania C, Costeffectiveness of implantable cardioverter-defibrillator in today’s worldIndian Heart Journal 2014 66(suppl-1):S101-04.10.1016/j.ihj.2013.12.03424568820 [Google Scholar] [CrossRef] [PubMed]
[36]. Shenthar J, Bohra S, Jetley V, Vora R, Lokhandwala A, Nabar Y, A survey of cardiac implantable electronic device implantation in India: by Indian society of electrocardiology and Indian heart rhythm societyIndian Heart Journal 2016 68(1):68-71.10.1016/j.ihj.2015.06.03726896270 [Google Scholar] [CrossRef] [PubMed]
[37]. Naik N, Juneja R, ICD for primary prophylaxis of sudden cardiac death : An Indian perspectiveJournal-Association of Physicians of India 2007 55:47-53. [Google Scholar]