Empyema thoracis is a significant cause for morbidity and mortality even in recent practice. A parapneumonic effusion develops in 50-70% of pneumonia patients, 20% of which may progress to empyema thoracis [1]. Novel approach with antibiotics and drainage may not suffice in empyema patients with loculations, fibropurulent or organising stage.
An open thoracostomy has been considered as gold standard for decortication in empyema patients; traditional thoracotomy is associated with increased postoperative pain and morbidity [2]. VATS has been established as safe and effective procedure for many thoracic surgeries and has shown to reduce pain and morbidity [3]; however, its role in the management of empyema thoracis remains which still needs to be studied. Studies have shown the efficacy of VATS in drainage of early fibropurulent phase (Stage 1) empyemas [4-8]. However, there are concerns regarding efficacy of VATS to adequately decorticate the lung and its role in later organising phase has still yet to be learnt [9-10].
In 1962, the American Thoracic Society described the formation of parapneumonic effusion as a continuous process, from the early exudative phase (Stage I) through the intermediate fibrinopurulent phase (Stage II) to finally the organising phase (Stage III) [11]. The incidence of empyema thoracis is found to be increasing along with changing microbial flora.
Empyema thoracis is among the few conditions where the management depends much on the appropriate timing of treatment in the course of the disease.
“A person with empyemata … shall die on the fourteenth day, unless something favourable supervenes” [12]
The principles of management of empyema thoracis have been known since the time of Hippocrates. The dictum “UBI Pus Evacua” ‘pus anywhere in the body, needs to be drained’ holds true even today.
Hence, it’s important to accurately stage the disease, using both clinical features as well as the findings on imaging. Central to staging scheme is the development of restrictive pleural peel encasing the lung [Table/Fig-1].
Despite the widespread use of antibiotics, empyema remains a common and serious problem, and its treatment is controversial. Various treatment options exist including intravenous antibiotics with repeated thoracocentesis, chest tube drainage, fibrinolytic therapy, VATS and thoracotomy with decortication [10,13-23]. There is no consensus on the ideal treatment or even the ideal timing of different options available. We felt some guidelines according to the staging of the disease for the appropriate mode of management would improve the outcome and decrease the morbidity. Hence, we undertook the study for the evaluation of role of VATS in the management of empyema thoracis.
The present study aims to compare surgical decortication for empyema thoracis performed using VATS and thoracostomy approaches assesed in terms of radiologic resolution, functional improvements and postoperative pain.
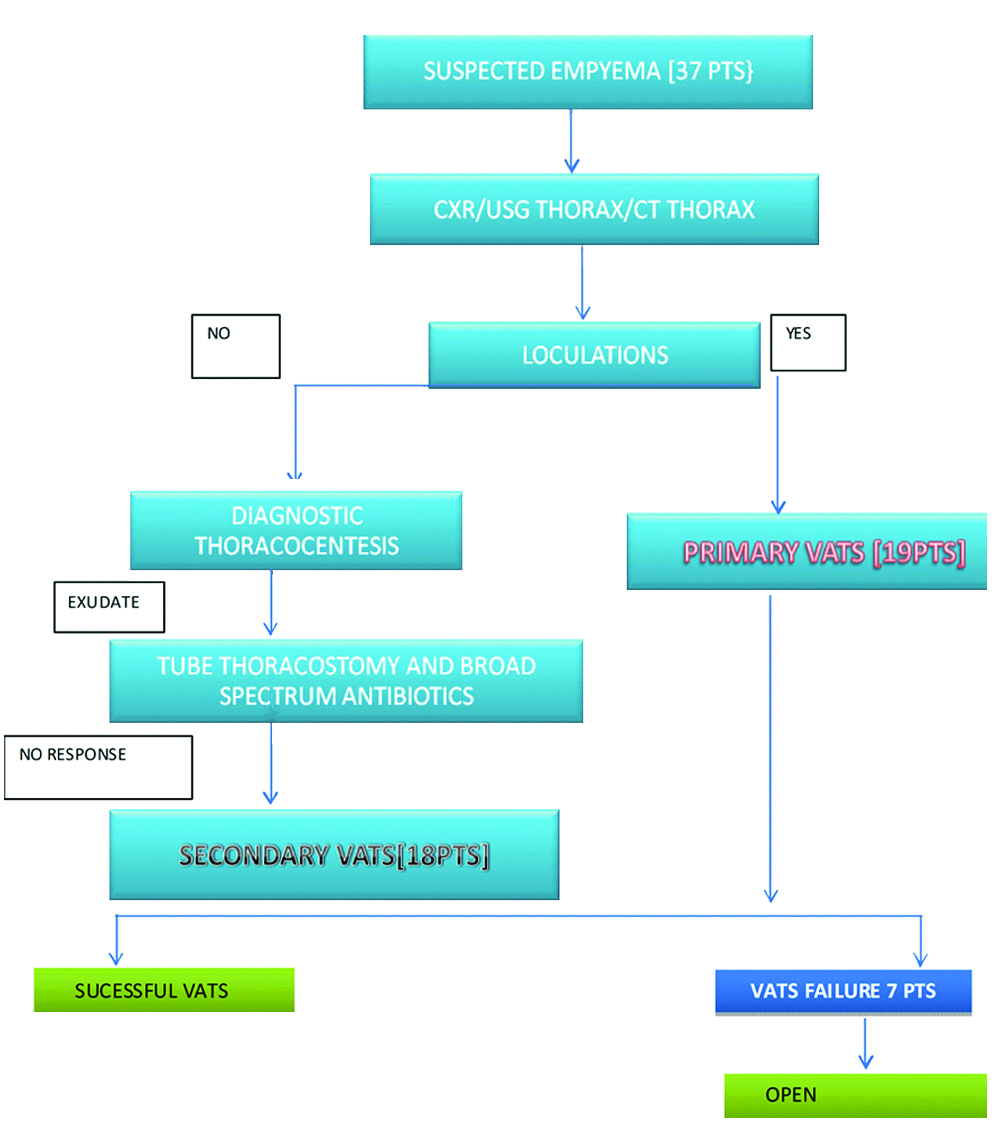
Role of primary VATS and secondary VATS (after an initial tube thoracostomy trial) in Stage I empyema with loculations.
Materials and Methods
A prospective study conducted in the Department of Surgery, Kempegowda Institute of Medical Sciences, Bengaluru, Karnataka, India, with purposive sampling which included 37 cases, who fulfilled the criteria. The duration of study, was 24 months October 2011 to October 2013, which included six months of follow up. A well informed consent was taken prior to study from all patients.
Inclusion criteria: Patients with empyema thoracis who failed to respond to medical treatment or chest tube drainage and patient with multiloculated empyema as diagnosed by CT scan or USG were included.
Exclusion criteria: Patient with tuberculous empyema, poor cardiac functioning and unfit for anaesthesia were excluded.
Tuberculous empyema is associated with more complications and recovery is delayed compared to bacterial empyema [24]. Hence, tuberculous empyema was excluded from present study.
Detailed history of each patient was taken and the patients were divided into three groups depending on the duration of symptoms. The groups denoted the stages of empyema as shown in [Table/Fig-2].
| Stage of empyema | Duration of symptoms |
|---|
| Stage I (exudative) | Less than 5 days |
| Stage II (fibrinopurulent) | 6 to 15 days |
| Stage III (organising) | More than 15 days |
Respiratory system was examined. Complete blood count was sent for all patients. In patients with suspicion of tuberculous mantoux test, blood Adenosine Deaminase (ADA) and Arterial Blood Gas (ABG) estimation was sent.
The CT scan of chest was done only in selected paediatric and adult cases, to assess the severity of the disease, the pleural thickness and parenchymal abnormalities such as abscess, necrotic tissue or pneumatoceles and to rule out any underlying malignancy, especially in elderly patients. Diagnostic thoracocentesis was done in all the patients under ultrasound guidance. The pleural fluid sample was sent for cytology and biochemical analysis, Ziehl-Neelsen (ZN) and Gram staining, culture sensitivity, adenosine deaminase levels.
A diagnosis of empyema thoracis was made in these children either by aspirating frank pus with WBC count >500/μL and increased polymorphs i.e., >50% or by demonstrating exudative nature of the pleural fluid according to Light’s criteria or by demonstrating organisms by Gram’s staining or by culture [25].
The ADA levels in pleural fluid >40 IU/L or positive Acid Fast Bacilli (AFB) were strongly suggestive of tuberculous empyema and those patients were excluded from the study [26].
Pulmonary Function Test (PFT) was done in all adult patients to assess preoperative Forced Expiratory Volume (FEV1) and its predictive value.
Treatment Modality
All the patients were started on broad spectrum antibiotics and bronchodilators with supportive oxygen inhalation. Antibiotics were changed according to the culture/sensitivity reports. Antipyretics and analgesics were given if required “any stage of empyema (clinically) without any loculations (radiologically) were treated initially with ICD tube thoracostomy.”
Video Assisted Thoracoscopic Surgery
In the present study, the indications of VATS were any stage of empyema (clinically) but showing multiloculations or thickened pleura on imaging were subjected to primary VATS.
Secondary VATS was indicated under the following circumstances, in patients who underwent initial ICD tube thoracostomy:
Failure of the lung to expand (evident on chest X-ray).
Worsening of the clinical condition of the patient (worsening symptoms, drop in oxygen saturation, raising leucocyte counts and respiratory acidosis).
Persistence of empyema or failure of adequate drainage due to thicker consistency of the purulent material.
Technical Details
The VATS was done under general anaesthesia with patient in lateral decubitus position. All cases were operated by a single surgeon. Single lumen endotracheal tube was used where there was hardly any expansion of the affected lung and the cavity was large to ensure no damage to the underlying lung. Double lumen endotracheal tube was used when the affected lung was not collapsed.
In present study, the first incision of 1-1.5 cm was usually placed between 4th to 7th intercostal space in mid axillary line and 10 mm trocar inserted; 10 mm (zero degree) video thoracoscope inserted and the working ports (5 or 6 mm) were inserted under vision to achieve triangulation and facilitate manipulation of the instruments. Tissue/fluid was sent for culture/sensitivity and histopathological analysis. Loculations were broken and plaques were removed using both blunt and sharp dissections. Special attention was given to clear the costophrenic sulcus in order to prevent adhesion related impairment of diaphragmatic mobility [Table/Fig-3].
If lung expansion was found to be inadequate after debridement then decortication was done. Additional ports were inserted to aid decortication. If decortication was also found to be inadequate, procedure was converted to open thoracostomy. The cavity was thoroughly inspected for any leaks or bleeding or any injury to the lung parenchyma [Table/Fig-4].
Usually an ICD tube was inserted through the first port and secured and connected to water seal drainage bag.
Dense plaques which could not be debrided by VATS, thickened pleura which could not be decorticated by VATS and technical difficulties were indications for open thoracotomy in the present study.
Postoperative Management
Endotracheal intubation was maintained in most of the patients. Patients were either shifted to paediatric ICU or surgical ICU and were connected to ventilator under sedation. Intravenous antibiotics and bronchodilators were continued. Weaning was done gradually and all the patients were extubated by postoperative day 1. Adequate analgesics (parenteral paracetamol infusion was the drug of choice) were administered.
Chest X-ray was done on postoperative day 1 and day 4 in all the patients. ICD was retained till effusion persisted and was removed if the following criteria were satisfied: no effusion, good lung expansion and fluid drained <50 mL/24 hours.
The PFTs was repeated after four weeks to assess FEV1. The percentage of predicted value was calculated and improvement in FEV1 was assessed to determine the lung expansion.
Pain Assessment
Pain assessment after surgery was done using Visual Analogue Scale (VAS) scoring in patients of age six years and above. Pain was assessed on postoperative day 1 and postoperative day 7 in all the patients.
Children’s Hospital of Eastern Ontario Pain Scale grading was used to assess pain in children of age less than six years.
Results
The mean age of presentation in paediatric group was 6.2 years and in adult group it was 51.8 years. Age ranged from 2-75 years. The mean hospital stay was 13.0 days (range: 5 to 30 days). The range of duration of surgery was from 54-192 minutes with a mean duration of 113.6 minutes [Table/Fig-5].
| Age in years | Number of adults | Percentage (%) |
|---|
| 29-30 | 1 | 6.3 |
| 31-50 | 4 | 25.0 |
| 51-70 | 10 | 62.5 |
| >70 | 1 | 6.3 |
| Total | 16 | 100.0 |
Fever was the most common complaint followed by cough and then breathlessness. A total of 13 patients were in Stage 1 (exudative phase), 15 patients of Stage 2 (fibro purulent phase) and 9 patients of (organisation) were in Stage 3 empyema [Table/Fig-6].
| Clinical stage of the disease | Duration of symptoms | Children | Adults | Total |
|---|
| Stage I | 1-5 | 6 (28.6%) | 7 (43.8%) | 13 (35.1%) |
| Stage II | 6-15 | 11 (52.4%) | 4 (25%) | 15 (40.5%) |
| Stage III | >15 | 4 (19%) | 5 (31.3%) | 9 (24.3%) |
| Total | 21 (100%) | 16 (100%) | 37 (100%) |
| Mean±SD | 9.43±4.69 | 11.25±8.53 | 10.22±6.58 |
The stages of the disease did not depend on the age of the patient.
Out of 37 patients, 19 patients were subjected to primary VATS depending upon radiological findings such as mulitiloculated empyema, restricted pleural peel.
Figure Protocol of Study and Procedure Done
A total of 18 patients were subjected to VATS after failed ICD thorocostomy.
Out of which 30 patients underwent successful VATS, 23 patients underwent VATS debridement and seven patients underwent VATS decortication successfully without any intraoperative complications, seven patients were converted to open thoracotomy due to failed VATS (inadequate debridement, technical difficulties) [Table/Fig-7].
Figure protocol of study and procedure done.
Pneumococcus is the most common cause of empyema in children and Staphylococcusaureus is the most common cause of empyema in adults. Aetiology was polymicrobial in three cases. In two cases fungal culture was positive.
There was a significant decrease in VAS scores on postoperative day 1 {F(1,24)=26.14, p<0.001} and day 7 {F(1,24)=28.09, p<0.001) in VATS group compared to thoracotomy group using one way ANOVA [Table/Fig-8].
Pain according to VAS scores.
| Paediatric | VAS on POD1 | VAS on POD7 | Improvement in (%) |
|---|
| Mean | 4.956 | 1.567 | 33.89 |
| Std. Deviation | 1.5461 | 0.9811 | 7.849 |
| Number | 9 | 9 | 9 |
| Adult |
| Mean | 5.225 | 1.800 | 34.25 |
| Std. Deviation | 1.4350 | 1.0973 | 8.331 |
| Number | 16 | 16 | 16 |
| Total |
| Mean | 5.128 | 1.716 | 34.12 |
| Std. Deviation | 1.4496 | 1.0423 | 7.996 |
| Number | 25 | 25 | 25 |
POD-Postoperative day, Std.-Standard, VAS-Visual analogue scale
Mean duration of postoperative ICD tube was 7.27 days (range: -4 to 16 days).
There is no statistical significance (p=0.79) in postoperative lung expansion between patients who underwent VATS and open thoracotomy.
Most of the patients had poor lung function (mean FEV1 65.25% of predicted value) who were diagnosed with empyema thoracis, before surgery. The lung function was good in most of the cases when FEV1 was assessed four weeks after surgery (mean 85.4% of predicted value).
There was statistically no difference in outcome with respect to FEV1 between patients who had successful VATS and who underwent open thoracotomy (p=0.6). Hence, both the procedures are equally effective with respect to the assessment of lung function.
A total of 9 patients who were in Stage 3 empyema out which 7 were succcesfully decorticated by VATS [Table/Fig-9].
Total various procedures according to stage of empyema.
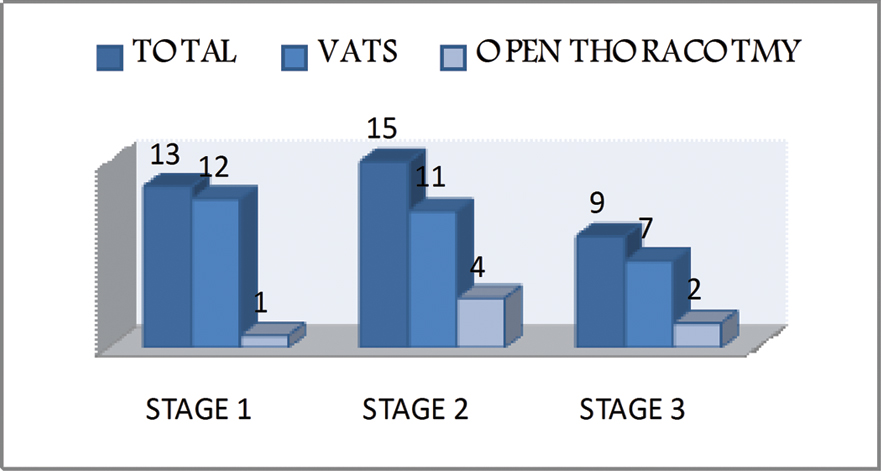
Hence, open thoracotomy should only be considerd for Stage 3 empyema only when VATS fails.
Early primary VATS in Stage I disease with multiloculations has better outcome with respect to lung expansion (p=0.049) and reduced hospital stay (p=0.08) compared to initial ICD tube thoracostomy followed by VATS (secondary VATS). There were no major intraoperative complications during VATS.
Discussion
The aim of surgical treatment for fibropurulent and organised phases of empyema thoracis is to drain all loculated collections and enable full lung re-expansion by removal of pleural peel from the lung surface.
Antibiotics, pigtail insertion, and tube thoracostomy still play acceptable roles in the initial management of empyema. However, by these methods, minimal success is reported that (36-65%) of the patients are not cured, even with a prolonged hospital stay and morbidity [27-29].
VATS, a procedure that is more effective than tube drainage, is an attractive alternative for treating empyema. Age, malignancy, chronic lung disease, chronic renal insufficiency, liver cirrhosis, polymicrobial infection, and positive bacterial culture are significant risk factors for mortality, while VATS can be performed on patients with all the comorbidities without much mortality [30].
In present study, all surgeries were done by a single experienced surgeon. Satisfactory results can be achieved with the VATS approach regardless of the phase of empyema. Although, with less experience we suggest conversion to open thoracotomy as same results can be achieved.
Main reason for failure of conservative methods is multiloculations, dense adhesion and restrictive pleural peel. Because of dense adhesions and technical difficulties in 7 cases (4 paediatric cases and 3 adult cases), conversion to open thoracotomy was done. Conversion to open thoracotomy was neither dependent on the age of the patient nor the stage of empyema (p=0.58). Further, in present study VATS decortication was done successfully in seven patients, out of which five were in Stage III empyema.
Patients with Stage I empyema who were subjected to primary VATS had shorter hospital stay, though statistically insignificant (p=0.08) and better outcome with respect to chest expansion (p=0.049) compared to those who were treated initially with ICD tube thoracostomy. Hence, early primary VATS is effective in patients with Stage I empyema proven to be multiloculated or thickened pleura, otherwise ICD tube thoracostomy should always be the first interventional treatment and VATS is to be reserved for those who fail to respond to initial ICD.
Conversion to open thoracotomy was done in seven cases of which two cases were of Stage III, four cases were Stage II and 1 case was Stage I. Thoracotomy leads to greater morbidity (pain) and prolonged hospital stay compared to VATS and should be done if VATS fails. However, there was no difference in the lung function postoperatively between VATS and open thoracotomy with respect to the FEV1 (p=0.6) and hence both are effective procedures.
The primary finding in this study was that both the thoracotomy and VATS approaches gave equally effective treatment for empyema in terms of radiologic and functional outcomes, even when patients with organising phase disease were included in the comparison.
The secondary finding was that VATS may be better accepted by patients. This study adds empyema decortication to the growing list of operations for which VATS is proven to give significantly less immediate postoperative pain than open thoracotomy.
We had compared present studies with various other studies, this study was unique study taking all 3 Stages of empyema and emphasising that even in Stage 1 empyema there can be loculations, by treating it conservatively many fail and subsequently progress to Stage 2/3 empyema [Table/Fig-10].
Comparison with other studies.
| Authors | n | Stage | Chest tube (days) | Hospital stay (days) | Success (%) | Conversion (%) | Complications (%) | Mortality (%) |
|---|
| Striffeler H et al., [6] | 67 | II | 4.1 | 12.3 | 72 | 28 | 4 | 4 |
| Angelillo Mackinley TA et al., [26] | 31 | II | 4.1 | 6.7 | 90 | 10 | 16 | 3 |
| Landreneau RJ et al., [27] | 76 | II/III | 3.3 | 7.4 | 83 | 17 | 3 | 6.6 |
| Ridley PD and Braimbridge MV [28] | 30 | II/III | 10.7 | - | 60 | 40 | - | 13 |
| Wait MA et al., [29] | 11 | II | 5.8 | 8.7 | 91 | 0 | 0 | 9 |
| Tsai CH et al., [30] | 33 | - | 15.6 | 27.5 | 75 | 6.2 | 7.2 | 3 |
| Present study | 37 | I/II/III | 7.27 | 13.0 | 81 | 19 | 17 | 0 |
Comparison with other Studies
However we have kept chest tube for a longer duration (mean 7 days postoperative) until we were satisfied with lung expansion. We had no mortality in present study, five patients had minor complications after VATS. Three of them had uncomplicated pleural effusion out of which two patients were treated with ICD tube drainage and the other by therapeutic percutaneous aspiration; two patients had surgical site infection and were treated with antibiotics according to culture/sensitivity report.
Conclusion
Video assisted thoracoscopic surgery is a safe and effective procedure to treat empyema thoracis, when tube thoracostomy fails or if the empyema is loculated with dense pleura. All the stages of empyema can effectively and safely be treated by VATS. Empyema at later stages can be treated effectively with VATS. Open thoracotomy should be reserved when VATS fails as thoracotomy, as it leads to more morbidity and prolongs the hospital stay.