Spink1 Mutation in Idiopathic Recurrent Acute Pancreatitis- A Pilot Study
Shiran Shetty1, Thaiagarajan Sairam2, Krishnaveni Janarthanan3, Venkatakrishnan Leelakrishnan4, Ramalingam Sankaran4
1 Associate Professor, Department of Gastroenterology and Hepatology, KMC Manipal, Manipal, Karnataka, India.
2 Associate Professor, PSG Center for Molecular Medicine and Therapeutics, PSG IMSR, Coimbatore, Tamil Nadu, India.
3 Professor, Department of Gastroenterology and Hepatology, PSG IMSR, Coimbatore, Tamil Nadu, India.
4 Professor, Department of Gastroenterology and Hepatology, PSG IMSR, Coimbatore, Tamil Nadu, India.
5 Professor, PSG Center for Molecular Medicine and Therapeutics, PSG IMSR, Coimbatore, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shiran Shetty, Associate Professor, Department of Gastroenterology, 4th Floor, New OPD Building, Kasturba Hospital, Manipal-576104, Karnataka, India.
E-mail: drshiran@gmail.com
Introduction
Recurrent Acute Pancreatitis (RAP) and Chronic Pancreatitis (CP) are labeled as idiopathic when no identifiable factors are found. The identification of genetic mutations associated with pancreatitis have provided opportunities for identifying patients at risk for idiopathic pancreatitis.
Aim
The aim of the present study was to study the clinical profile and prevalence of SPINK1 mutation in idiopathic RAP.
Materials and Methods
The present study was a prospective observational study of idiopathic RAP patients at a tertiary care hospital. DNA was isolated from blood obtained from patients and genotyping of SPINK1 mutation was studied.
Results
A total of 17 patients with idiopathic RAP were included. Their mean age was 22.29±9.7 years, 14 (82%) were male, 7 (41.7%) had SPINK1 mutation. Patients with SPINK1 mutations had more frequent acute episodes of pancreatitis.
Conclusion
SPINK1 mutation patients have more frequent acute episodes of pancreatitis and nearly 7 (41.7%) had SPINK1 mutations in the so called idiopathic RAP. Genetic testing in idiopathic pancreatitis might have role in future. Multicenter studies are further required to confirm the role of genetic testing in RAP.
Gastroenterology, Gene mutation, Prevalence
Introduction
Pancreatitis is the occurrence of inflammation in an otherwise normal gland and is commonly seen when patients present with severe abdominal pain. It is diagnosed by appropriate imaging and with the help of laboratory markers viz., Pancreatic amylase and lipase [1]. Acute pancreatitis can contribute to the development of RAP if aetiology remains unknown. The RAP is defined as two or more attacks of acute pancreatitis with symptom free intervals. Evaluation and appropriate treatment is vitalin RAP since >50% of patients experience recurrent episodes that contributes to the development of CP [2,3]. In clinical practice routine evaluation often fails to detect the cause of pancreatitis in 10-30% of the patients, and these patients are referred as idiopathic chronic or RAP [4]. A very few studies have been conducted on idiopathic RAP. The RAP can be due to biliary disease, alcohol, trauma, hypercalcemia, hyperlipidemia, or anatomical variations [3,4].
Mutations in Cationic Trypsinogen gene (PRSS1), SPINK1 gene, Cystic Fibrosis Transmembrane Conductance Regular (CFTR) gene and Cathepsin B gene have been studied in RAP and CP [5-8]. The SPINK1 is also known as pancreatic secretory ‘Trypsin’ inhibitors located on chromosome 5. It is a 56 amino acid peptide that inhibits Trypsin by blocking the active site, provides the first line defense against premature Trypsinogen activation within pancreas, because it is capable of inhibiting about 20% of Trypsin activity by competitively blocking the active site of trypsin [7,8]. Recently, the role of SPINK1 mutation in CP has emerged. The most frequent mutation in SPINK1 gene exon 3 results in asparagine to serine amino acid change (N34S) which leads to decreased trypsin inhibitory capacity. SPINK1 (N34S), mutations are relatively common in 2% of general population, shown to be associated with pancreatitis [9]. SPINK1 mutation is hypothesised to be a susceptibility factor or a disease modifier in recurrent pancreatitis [10].
Genetic mutations may be the cause of pancreatitis in patients where aetiology remains undiagnosed [Table/Fig-1] [11,12]. Idiopathic pancreatitis represents a complex disease process resulting from an interaction of genetic mutations and environmental factors. Recent research have shown complex interactions like gene-gene, gene-environment in the pathogenesis of pancreatitis [13]. A small pilot systematic study on clinical profile and prevalence of SPINK1 mutation was studied in patients with idiopathic RAP.
Progression from pre-acute to chronic pancreatitis.
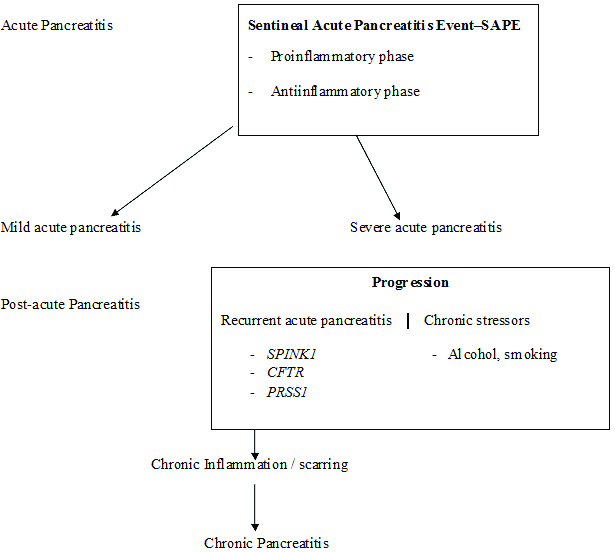
In the present study we aim to study the demographic and clinical profile of idiopathic RAP and to assess the prevalence of genetic mutation (SPINK1) in idiopathic RAP.
Materials and Methods
The study protocol was approved by the Institutional Human Ethics Committee (IHEC) prior to the start of the study. Written informed consent was obtained from patients prior to study entry. A total of 17 patients who met the inclusion criteria of idiopathic RAP were enrolled. Since the disease is rare, we wanted to include all possible cases, hence no sample size was separately determined.
Inclusion Criteria
The inclusion criteria was selected on the basis of prior studies [9,11,12]. Two documented episodes of typical pancreatic type of abdominal pain, amylase or lipase greater than three times the upper limit of normal, features of acute pancreatitis on imaging studies (ultrasound/CT abdomen), no identifiable cause or risk factors.
Exclusion Criteria
Patients with identifiable cause and risk factors for acute and recurrent pancreatitis, malignancy, retroviral infection, psychiatric illness, hereditary and known genetic diseases.
Work up: All patients underwent complete blood counts, biochemical investigations including liver function test, renal function, fasting blood sugar, serum calcium, lipid profile, serum amylase and lipase, antinuclear antibody, endocrine work up Ig4 levels viral serology and Endoscopic Ultrasound (EUS) for microlithiasis and sludge after informed consent. The following imaging studies viz., transabdominal ultrasonography, Contrast enhanced CT abdomen, Magnetic resonance cholangiopancreatography/EUS and SPINK1 mutation testing were done.
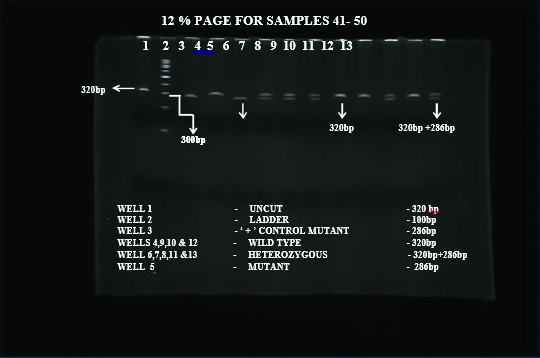
Genotyping: Genomic DNA was extracted from blood using QIAamp DNA Blood Mini Kit (Qiagen, USA). Genotyping initiated with DNA extraction, followed by Polymerase Chain Reaction (PCR) to amplify SPINK1 gene, RFLP using PstI restriction enzyme and Poly Acrylamide Gel Electrophoresis (PAGE) of the digested product [14]. The PCR reactions were performed on Eppendorf thermocycler. Primers used were forward primer-SPINKR: TTCTGTTTAATTCCATTTTTAGGCCAAATGCTGCA; reverse primer-SPINKR: GGCTTTTATCATACAAGTGACTTCT. DNA was isolated using Micro AX Blood Gravity Kit (A&A Biotechnology, Gdańsk, Poland). Quality and concentration of isolated DNA was measured by Nano Drop 2000 (Thermo Scientific, TK Biotech, Poland). Genotyping was performed using Allele-Specific PCR (ASA-PCR) and High-Resolution Melting (HRM)-PCR as described previously [15]. The genotypes were determined based on the expected product size [Table/Fig-2].
Genotypes with their expected product size.
| Genotype | Mutation Site | Product Size (bp) |
|---|
| A/A | (Asn 34 Asn) | 320 bp |
| G/G | (Ser 34 Ser) | 286 bp |
| A/G | (Asn 34 Ser) | 320, 286 and 34 |
Asn: Asparagine; Ser: Serine; bp: Base pairs
Statistical Analysis
Study variables were analysed by statistical package SPSS (Version 10.0. Descriptive statistics mean and standard deviation were used, number and percentage are given where appropriate.
Results
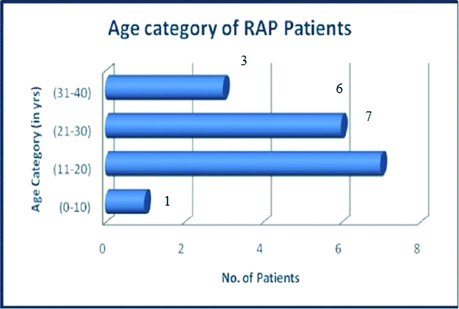
A total of 17 patients were included and their mean age was 22.29±9.70 years, the youngest enrolled person was 14-year-old and the oldest was 39-year-old with 14 patients below 30 years of age [Table/Fig-3]. The duration of illness was 28.23±10.34 months and mean fasting glucose level was 91.64±15.94 mg/dL. The following graph represents age category. A total of 14 (82%) patients were male and 3 (18%) were female, suggesting male preponderance. Majority of the patients had BMI >18.5 kg/m2.
Age among RAP patients at enrollment.
Majority of patients had three episodes of pancreatitis [Table/Fig-4]. Patients with SPINK1 mutation positive had more number of pancreatitis episodes compared to wild type. All the SPINK1 positive patients had four and above pancreatitis episodes. Out of 17 patients with idiopathic RAP 7 (41.17%) were positive for SPINK1 mutation. A total of 10 (58.3%) had wild type and 7 (41.7%) had heterozygous genotype [Table/Fig-5a,b].
Pancreatitis episodes among RAP with SPINK1 mutation.
| Pancreatitis Episodes | No. of Patients | No. of SPINK1 positive |
|---|
| ≤3 | 8 | - |
| 3-4 | 2 | 5 |
| 4-5 | - | 1 |
| > 5 | - | 1 |
PCR gel image depicting bands of various phenotypes and genotypes.
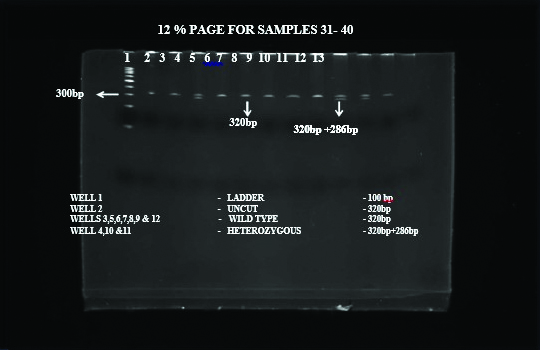
Heterozygous and wild SPINK1 mutation on PCR gel image.
Discussion
Acute pancreatitis can lead to recurrent episodes and cause CP. In practice many a times no cause can be made out and such cases are labeled as idiopathic pancreatitis. Many studies are focused on idiopathic CP; however, only few have been on truly idiopathic recurrent pancreatitis, so we conducted a short pilot study. The present study was a prospective analysis of 17 patients diagnosed to have idiopathic RAP. The present study was conducted on South Indian population to assess SPINK1 mutation and clinical profile in idiopathic RAP. Male dominance was evident as seen in two prior studies around the world [17,18]. It is currently unknown as to why this is more commonly seen in male patients. The mean age of the patients in two prior studies were 41 and 43 years, respectively [16,17]. while, the mean age of the patients in present study was 22.29 years. All patients in the present study had recurrent episodes of pancreatitis (>2) which was similar to a North Indian study [18]. The present study had 17 patients out of which 7 (41.17%) were positive for SPINK1 mutation and all seven patients had increased episodes of pancreatitis compared to wild type (no mutation). Another study by Wkitcomb DC, shown significant cases of idiopathic RAP have genetic components like SPINK1, CFTR [19]. Genetic theory on pancreatitis says that carrying SPINK1 mutation leads to increased episodes of acute pancreatitis and leads to CP later [20,21]. However, another study by Cavestro GM et al., did not find a statistically significant association with this mutation [22]. Hence there is a need to assess additional mutations which could be present in addition to SPINK1 mutation.
Genetic mutations seem to play an important role in the pathogenesis of idiopathic CP. Recent studies have shown a role of Chymotrypsin C gene mutation in idiopathic CP which lands support to the genetic theory of aetiopathogenesis of idiopathic CP. The present study revealed that SPINK1 mutation is strongly associated with more number of acute episodes in idiopathic RAP. We need to carry out the present study in more number of patients and replicate results for confirmation. Also, it may be useful to do functional studies of SPINK1 mutation in cell cultures to understand the pathophysiology of disease status more completely. Molecular and genetic analysis will become important in future. Mutation will provide information on risk of developing pancreatitis. Mutation detection will assist in early diagnosis, mutation identified will provide rationale classification. Molecular classification will help in knowing disease progression and prognosis. Specific mutation will help in gene-environmental interaction. Mutation may help in developing new therapeutic intervention. Such studies will help patients to understand as to why some develop pancreatitis while rest don’t.
Limitation
Being single centered with a smaller sample size limits generalisability of our findings. Limitation also arises from the selected study approach as to not include mutations other than SPINK1.
Conclusion
By prospectively analysing RAP patients in a tertiary care center, we studied SPINK 1 mutation. SPINK 1 mutation patients have frequent episodes of pancreatitis and two fifth of the study population had SPINK 1 mutations. Genetic testing has an evident role in future for idiopathic RAP. The need of the hour is to have genetic mutational studies at various centers with a bigger sample size to identify new mutations.
[1]. Pujahari AK, Chronic Pancreatitis: A reviewIndian J Surg 2015 77(Suppl 3):1348-58.10.1007/s12262-015-1221-z27011563 [Google Scholar] [CrossRef] [PubMed]
[2]. Schneider A, Whitcomb DC, Hereditary pancreatitis: a model for inflammatory disease of the pancreasBest Pract Res Clin Gastroenterol 2002 16(3):347-63.10.1053/bega.2002.031112079262 [Google Scholar] [CrossRef] [PubMed]
[3]. Somogyi L, Martin SP, Venkatesan T, Ulrich CD 2nd, Recurrent Acute Pancreatitis: An algorithmic approach to identification and elimination of inciting factorsGastroenterology 2001 120(3):708-17.10.1053/gast.2001.2233311179245 [Google Scholar] [CrossRef] [PubMed]
[4]. Levy MJ, Geenen JE, Idiopathic acute recurrent pancreatitisAm J Gastroenterol 2001 96(9):2540-55.10.1111/j.1572-0241.2001.04098.x11569674 [Google Scholar] [CrossRef] [PubMed]
[5]. Sobczyńska-Tomaszewska A, Bak D, Oralewska B, Oracz G, Norek A, Czerska K, Analysis of CFTR, SPINK1, PRSS1 and AAT mutations in children with acute or chronic pancreatitisJ Pediatr Gastroenterol Nur 2006 43(3):299-306.10.1097/01.mpg.0000232570.48773.df16954950 [Google Scholar] [CrossRef] [PubMed]
[6]. Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Hereditary Pancreatitis is caused by a mutation in the cationic trypsinogen geneNat Genet 1996 14(2):141-45.10.1038/ng1096-1418841182 [Google Scholar] [CrossRef] [PubMed]
[7]. Funakoshi A, Miyasaka K, Jimi A, Kitani K, Teraoka H, Yoshida N, Protective effect of human pancreatic secretory tyrpsin inhibitors on cerulean-induced acute pancreatitis in ratsDigestion 1992 52(3-4):145-51.10.1159/0002009461459347 [Google Scholar] [CrossRef] [PubMed]
[8]. Tukiainen E, Kylänpää ML, Kemppainen E, Nevanlinna H, Paju A, Repo H, Pancreatic secretory tyrpsin inhibitor gene SPINK 1 mutations in patients with acute pancreatitisPancreas 2005 30(3):239-42.10.1097/01.mpa.0000157479.84036.ed15782101 [Google Scholar] [CrossRef] [PubMed]
[9]. Ravi Kanth V, Nageshwar Reddy D, Genetics of acute and chronic pancreatitis: An updateWorld J Gastrointest Pathophysiol 2014 5(4):427-37.10.4291/wjgp.v5.i4.42725400986 [Google Scholar] [CrossRef] [PubMed]
[10]. Whitcomb DC, Genetic Risk Factors for Pancreatic DisordersGastroenterology 2013 144(6):1292-302.10.1053/j.gastro.2013.01.06923622139 [Google Scholar] [CrossRef] [PubMed]
[11]. Khurana V, Ganguly I, Recurrent acute pancreatitisJ Pancreas 2014 15(5):413-26. [Google Scholar]
[12]. Atkinson AJJ, Colburn WA, DeGruttola VG, DeMcts DL, Downing GL, Hoth DF, Biomarkers and surrogate endpoints: preferred definitions and conceptual frameworkClin Pharmacol Ther 2001 69(3):89-95.10.1067/mcp.2001.11398911240971 [Google Scholar] [CrossRef] [PubMed]
[13]. Whitcomb DC, Going MAD: development of a matrix academic division “to facilitate translating research to personalized medicineAcad Med 2011 86(11):1353-59.10.1097/ACM.0b013e3182303d7a21952059 [Google Scholar] [CrossRef] [PubMed]
[14]. Shetty S, Leelakrishnan V, Krishnaveni Ramalingam R, Seethalakshmi A study of SPINK1 mutation and other clinical correlates in idiopathic chronic pancreatitisJ Pancreas 2016 17(6):607-11. [Google Scholar]
[15]. Diaconu BL, Ciobanu L, Mocan T, Pfützer RH, Scafaru MP, Acalovschi M, Investigation of the SPINK1 N34S mutation in Romanian patients with alcoholic chronic pancreatitis. A clinical analysis based on the criteria of the M-ANNHEIM classificationJ Gastrointestin Liver Dis 2009 18(2):143-50. [Google Scholar]
[16]. Gao YJ, Li YQ, Wang Q, Li SL, Li GQ, Ma J, Analysis of clinical features of recurrent acute pancreatitis in ChinaJ Gastroenterol 2006 41(7):681-85.10.1007/s00535-006-1820-316933006 [Google Scholar] [CrossRef] [PubMed]
[17]. Gullo L, Migliori M, Pezzilli R, Oláh A, Farkas G, Levy P, An update on recurrent acute pancreatitis: data from five European countriesAm J Gastroenterol 2002 97(8):1959-62.10.1111/j.1572-0241.2002.05907.x12190160 [Google Scholar] [CrossRef] [PubMed]
[18]. Garg PK, Tandon RK, Madan K, Is biliary microlithiasis a significant cause of idiopathic recurrent acute pancreatitis? A long-term follow-up studyClin Gastroenterol Hepatol 2007 5(1):75-79.10.1016/j.cgh.2006.06.02316931169 [Google Scholar] [CrossRef] [PubMed]
[19]. Whitcomb DC, Genetic predispositions to acute and chronic pancreatitisMed Clin North Am 2000 84(3):531-47.10.1016/S0025-7125(05)70238-8 [Google Scholar] [CrossRef]
[20]. Pfutzer RH, Whitcomb DC, SPINK 1 mutations are associated with multiple phenotypesPancreatology 2001 1(5):457-60.10.1159/00005584712120224 [Google Scholar] [CrossRef] [PubMed]
[21]. Whitcomb DC, Hereditary Pancreatitis: New insights into acute and chronic pancreatitisGut 1999 45(3):317-22.10.1136/gut.45.3.31710446089 [Google Scholar] [CrossRef] [PubMed]
[22]. Cavestro GM, Zuppardo RA, Bertolini S, Sereni G, Frulloni L, Okolicsanyi S, Connection between genetics and clinical data: Role of MCP–I, CFTR, SPINK 1 in the setting of acute, acute recurrent and chronic pancreatitisAm J Gastroenterol 2010 105(1):199-206.10.1038/ajg.2009.61119844201 [Google Scholar] [CrossRef] [PubMed]