Baseline NT-ProBNP Level as a Risk Predictor of Contrast Induced-Acute Kidney Injury in Acute Coronary Syndrome Patients Undergoing Primary Angioplasty
Sumit Agarwal1, Hashir Kareem2, Tom Devasia3, Rameswer Reddy Mallu4, Ganesh Paramasivam5, Ajit Singh6, Prasad Narayan Shetty7, Suheil Dhanse8
1 Registrar, Department of Cardiology, Kasturba Medical College and Hospital, Manipal, Karnataka, India.
2 Associate Professor and Unit Head, Department of Cardiology, Kasturba Medical College and Hospital, Manipal, Karnataka, India.
3 Professor and Head, Department of Cardiology, Kasturba Medical College and Hospital, Manipal, Karnataka, India.
4 Registrar, Department of Cardiology, Kasturba Medical College and Hospital, Manipal, Karnataka, India.
5 Assistant Professor, Department of Cardiology, Kasturba Medical College and Hospital, Manipal, Karnataka, India.
6 Research Associate and PhD Scholar, Department of Cardiology, Kasturba Medical College and Hospital, Manipal, Karnataka, India.
7 PhD Scholar, Department of Cardiology, Kasturba Medical College and Hospital, Manipal, Karnataka, India.
8 Registrar, Department of Cardiology, Kasturba Medical College and Hospital, Manipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Hashir Kareem, Associate Professor, Department of Cardiology, Kasturba Medical College and Hospital, Manipal Academy of Higher Education, Manipal-576104, Karnataka, India.
E-mail: hashirkareem@gmail.com
Introduction
Contrast Induced Acute Kidney Injury (CI-AKI) is a common complication of invasive cardiovascular procedures. A three-fold higher risk of developing CI-AKI has been observed in patients with Acute Coronary Syndrome (ACS) who undergo Percutaneous Coronary Intervention (PCI). Several risk score models reflecting the cumulative risk of several peri-procedural predictors, such as the Mehran CIN score and Battle Management Command and Control (BMC2) CIN score, have been established and proven. Recent data suggest that baseline NT-proBNP may help to identify ST-Elevation Mycardial Infraction (STEMI) patients at risk for CI-AKI after primary PCI.
Aim
The aim of the present was to prognosticate ACS patients treated with Primary PCI for the risk of developing CI-AKI by correlating it with pre procedural NT-proBNP levels.
Materials and Methods
The present study was a prospective cross-sectional observational study, involving 150 patients with ACS undergoing PCI at Kasturba Medical College, Manipal, Karnataka, India (January 2016 to December 2016). Patients with ACS (STEMI and NSTEMI) who underwent primary PCI were included in the study. Pre-existing renal derangement, acute left ventricular failure and cardiogenic shock patients were excluded from the present study. Continuous variables were described as mean±standard deviation and compared by using the t-test or Wilcoxon rank-sum test. Categorical variables are described in terms of frequency and percentage and compared using the Chi-square or Fisher exact test. The Receiver Operating Characteristic (ROC) curves, paired sample t-test and independent t-test were applied for further analysis.
Results
A total of 150 patients (mean age, 63.03±9.07 years and 64.3% male) were included in the study. Among the study 22 (14.6%) patients developed CI-AKI. The value of NT-proBNP at presentation was significantly higher in patients who developed CI-AKI compared to those who did not (p<0.001). A cut off value of NT-proBNP of ≥2320 pg/mL as measured on admission has 90.9% sensitivity and 81.5% specificity in predicting CI-AKI.
Conclusion
Higher Baseline NT-proBNP levels (with a cut off value of 2620.46 pg/mL) can predict the development of CI-AKI after Percutaneous Transluminal Coronary Angioplasty (PTCA) in patient with ACS.
Glomerular filtration rate, Myocardial infarction, Percutaneous coronary intervention, ST-elevation myocardial infarction, Visipaque
Introduction
Most of the cases of Contrast Induced Nephropathy (CIN) are Iatrogenic mainly due to dye used in various diagnostic modalities. The CI-AKI is a common complication of invasive cardiovascular procedures. A three-fold higher risk of developing CI-AKI has been observed in patients with ACS who undergo PCI [1]. Several risk score models reflecting the cumulative risk of several peri-procedural predictors, such as the Mehran CIN score and BMC2 CIN score, have been established and proven [2]. Reliable and easily available markers are needed which identify patients likely to develop CI-AKI. Recent data suggest that baseline NT-proBNP may help to identify STEMI patients at risk for CI-AKI after primary PCI [3]. The NT-proBNP has been linked to haemodynamic imbalance, poor neurohormonal responses (like release of adenosine and endothelin causing renal vasoconstriction) and inflammation in ACS patients, which play a role in the development of CI-AKI [4]. It is important that parents at risk for developing CI-AKI should be identified early so that the treating physician can take the necessary precautions to prevent it [5]. The present study aimed to prognosticate ACS patients treated with Primary PCI for the risk of developing CI-AKI by correlating it with pre-procedural NT-proBNP levels.
Materials and Methods
The present study was a prospective cross-sectional observational study involving 150 patients with ACS (STEMI and NSTEMI) who underwent primary PCI at Kasturba Medical College, Manipal, Karnataka, India from January 2016 to December 2016 were included in the study. Pre-existing renal derangement, acute left ventricular failure and cardiogenic shock patients were excluded. Consent was taken from the subjects included in the study and Ethical approval was obtained from Institutional Ethics Committee.
Baseline serum creatinine and NT-proBNP levels were measured before the procedure. Nephrotoxic drugs were stopped on admission. The formula used for maximum contrast dose was [6]:
5 × body weight [kg])/serum creatinineThe Modification of Diet in Renal Disease (MDRD) equation was used to calculate Estimated Glomerular Filtration Rate (eGFR). The Simpson method and M mode was used for better assessment Left Ventricular Ejection Fraction (LVEF). Repeat Serum creatinine was measured at 48 hours after contrast medium administration. The CI-AKI was defined as a relative increase of ≥25% or absolute increase of ≥0.5 mg/dL in creatinine concentrations within 48 hours after index angiography [7].
The decision to use the trans-radial or trans-femoral approach was made by the concerned operator. On admission, all patients received a loading dose of 180 mg of Ticagrelor or 300 mg of clopidogrel in addition to 300 mg of Aspirin, 40 mg of Atorvastatin and 1.2 gm of N-acetyl cysteine. Normal saline was used as intravenous fluid during and after the procedure. Heparin dose was titrated to maintain an activated clotting time of around 300 seconds during procedure. The contrast agent used was Visipaque (non-ionic, isosmolar) which is an institutional policy. Thrombolysis in Myocardial Infarction grade 3 coronary (TIMI 3) flow with less than 30% residual stenosis was considered successful PCI.
Statistical Analysis
Continuous variables were described as mean±standard deviation and compared by using the t-test or Wilcoxon rank-sum test. Categorical variables were described in terms of frequency and percentage and compared using the Chi-square or Fisher exact test. The best cut off value of NT-proBNP for predicting CI-AKI was determined by the ROC curves analysis. Paired sample t-test was used to analyse haematological parameters at baseline and after 48 hours. Independent t-test was used to analyse difference in the mean values at presentation and at 48 hours after procedure. (p-value<0.001). SPSS version 20.0 was used for statistical analysis.
Results
A total of 150 patients (mean age, 63.03±9.07 years and 64.3% male) were included in the study. Baseline measurements of serum NT-proBNP and creatinine were taken. Creatinine was measured again at 48 hours. A total of 22 patients (14.6%) among the study population developed CI-AKI.
The baseline clinical characteristics of the patient population are summarised in [Table/Fig-1]. There were no significant differences in gender distribution between the groups. The prevalence of hyperlipidemia, hypertension, prior myocardial infarction, diabetes mellitus, prior stroke, prior medications, in hospital medications, and type of ACS (STEMI and NSTEMI) were not statistically different between the two groups. Patients in the CI-AKI group were older (73.12±12.29 years v/s 61.23±12.29 years respectively; p<0.001) and had a lower prevalence of smoking (p<0.001) compared to patients in the non-CI-AKI group. The CI-AKI group had lower LVEF when compared to the non-CI AKI group (p<0.001) with a higher number of patients with EF<40% in the former group.
Baseline characteristics of the study patients.
| Group | p-value |
|---|
| Mean±SD |
|---|
| Non CI-AKI(n=128) | CI-AKI(n=22) |
|---|
| Age (year) | 61.23±12.29 | 73.12±12.59 | <0.001 |
| Male gender, n (%) | 83 (64.8) | 14 (63.6) | 1.000 |
| BMI, kg/m2 | 28.55±4.33 | 28.13±5.47 | 0.687 |
| Systolic blood pressure (mmHg) | 130.0±24.5 | 134.1±32.2 | 0.491 |
| Diastolic blood pressure (mmHg) | 78.3±14.1 | 77.8±16.2 | 0.880 |
| Hypertension, n (%) | 55 (42.9) | 12 (54.5) | 0.358 |
| Diabetes mellitus, n (%) | 41 (32.0) | 8 (36.4) | 0.806 |
| Smoking, n (%) | 60 (46.8) | 3 (13.6) | 0.004 |
| Dyslipidema, n (%) | 38 (29.7) | 4 (18.1) | 0.315 |
| Prior CABG, n (%) | 7 (5.4) | 1 (4.5) | 1.000 |
| Prior myocardial infarction, n (%) | 12 (9.4) | 2 (9.0) | 1.000 |
| LVEF, % | 46.5±9.5 | 40.7±9.8 | 0.009 |
| LVEF <40% (%) | 20.9 | 38.5 | 0.008 |
| Type of ACS, n (%) | | | |
| STE-ACS | 80 (62.5) | 13 (59.1) | 0.814 |
| NSTE-ACS | 48 (37.5) | 9 (40.9) | |
| Treatment before admission, (%) | | | |
| Pts on ACEIs or ARBs | 28.2 | 31.4 | 0.756 |
| Pts on Statins | 28.4 | 27.2 | 1.000 |
ACS=Acute coronary syndrome; CABG=Coronary artery bypass graft; CI-AKI=Contrast induced acute kidney injury; LVEF=Left ventricular ejection fraction; NSTE=non-ST-segment elevation; STE=ST-segment elevation; BMI=Body mass index; ACEI=Angiotensin converting enzyme inhibitor; ARB=Angiotensin receptor blocker
The baseline laboratory measurements of the study population are shown in [Table/Fig-2]. It was noted that the patients in the CI-AKI group had significantly higher peak troponin T levels, a higher baseline serum creatinine value, and higher uric acid levels. The baseline haemoglobin, total cholesterol, Low Density Lipoprotein (LDL) and eGFR were lower in the CI-AKI group [Table/Fig-3,4].
Baseline biochemical and haematologic measurements of patients between groups.
| Group | p-value |
|---|
| Mean±SD |
|---|
| Non CI-AKI(n=128) | CI-AKI(n=22) |
|---|
| Serum glucose on admission | 157.1±87.5 | 156.5±76.6 | 0.975 |
| HbA1c, (%) | 7.11±1.9 | 6.95±2.0 | 0.717 |
| eGFR, mL/minute per 1.73 m2 | 72.4±18.9 | 51.1±16.7 | <0.001 |
| White blood cell count, × 103/mm3 | 10.59±4.51 | 12.28±3.88 | 0.100 |
| Platelet count, × 103/mm3 | 241.5±65.6 | 247.3±81.2 | 0.492 |
| Mean platelet volume, Fl | 8.75±1.03 | 9.29±1.21 | 0.001 |
| Total cholesterol, mg/dL | 191.2±49.1 | 175.4±51.2 | 0.167 |
| Triglyceride, mg/dL | 133 (35-815) | 114 (39-624) | 0.454 |
| Low-density lipoprotein, mg/dL | 121.1±42.0 | 106.3±40.1 | 0.126 |
| High-density lipoprotein, mg/dL | 41.5±10.1 | 42.5±11.1 | 0.673 |
| hs-CRP, mg/L | 7.45±4.28 | 8.68±4.28 | 0.215 |
| Peak CK-MB, ng/mL | 35.6 (0.78-425) | 50.4 (2.25-419) | 0.318 |
| Peak troponin T, ng/mL | 1062 (4.13-10000) | 1782 (43.9-10000) | 0.029 |
CK-MB-Creatine kinase-myocardial band; eGFR-Estimated glomerular filtration rate; HbA1c-Glycated haemoglobin; hs-CRP-High-sensitivity C-reactive protein; NT-proBNP-N-terminal pro-brain natriuretic peptide; inside the bracket the values denotes the lowest and the highest in the study population
Paired sample t-test: haematological parameters at baseline and after 48 hours.
| Characteristics (n=150) | At presentation | After 48 hours | p-value |
|---|
| Mean±SD | Range | Mean±SD | Range | |
|---|
| Urea | 26.01±10.3 | (8-72) | 28.59±14.4 | (10-133) | 0.075 |
| Creatinine (mg/dL) | 1.02±0.3 | (0.5-2.1) | 1.16±0.3 | (0.7-2.2) | <0.001 |
| Hb (g/L) | 13.58±2.2 | (7.3-23.0) | 12.51±2.2 | (5.1-21.4) | <0.001 |
| NT-proBNP (pg/mL) | 2620.46±4073.3 | (11.6–34637.0) | - | - | - |
HB-Haemoglobin; NT-proBNP=N-Terminal pro brain natriuretic peptide
Inside the bracket the values denotes the lowest and the highest in the study population
Independent t-test showing difference in the mean values at presentation and at 48 hours after procedure.
| | AKI | p-value |
|---|
| Mean±SD |
|---|
| Non AKI (n=128) | AKI (n=22) |
|---|
| Urea | At presentation | 26.38±10.5 | 23.86±9.5 | 0.294 |
| After 48 hours | 28.86±14.9 | 27.05±11.4 | 0.589 |
| Creatinine (mg/dL) | At presentation | 1.03±0.3 | 0.96±0.2 | 0.279 |
| After 48 hours | 1.11±0.3 | 1.48±0.2 | <0.001 |
| Hb (g/L) | At presentation | 13.53±1.9 | 13.95±3.1 | 0.403 |
| After 48 hours | 12.43±2.0 | 12.98±2.9 | 0.268 |
| NT-proBNP (pg/mL) | At presentation | 1088.29±2608.6 | 5043.99±3291.7 | <0.001 |
HB=Haemoglobin; NT-proBNP=N-Terminal pro brain natriuretic peptide
The value of NT-proBNP at presentation was significantly higher in patients who developed CI-AKI compared to those who did not {CI-AKI group: 5043.99±3291.7 (pg/mL), non-CI-AKI group: 1058.29±608.6 (pg/mL) respectively; p<0.001} as shown in [Table/Fig-3,4]. Frequency of CI-AKI in various age groups, haemoglobin ranges and left ventricular function (Ejection fraction) are shown in [Table/Fig-5].
Frequency of CI-AKI among the age groups, haemoglobin and left ventricular ejection fraction.
| Age Group | Frequency of CI-AKI |
|---|
| ≥60 | 8 |
| <60 | 14 |
| Haemoglobin | |
| >10 | 12 |
| <10 | 10 |
| LVEF | |
| >40 | 9 |
| <40 | 13 |
LVEF-Left ventricular ejection fraction
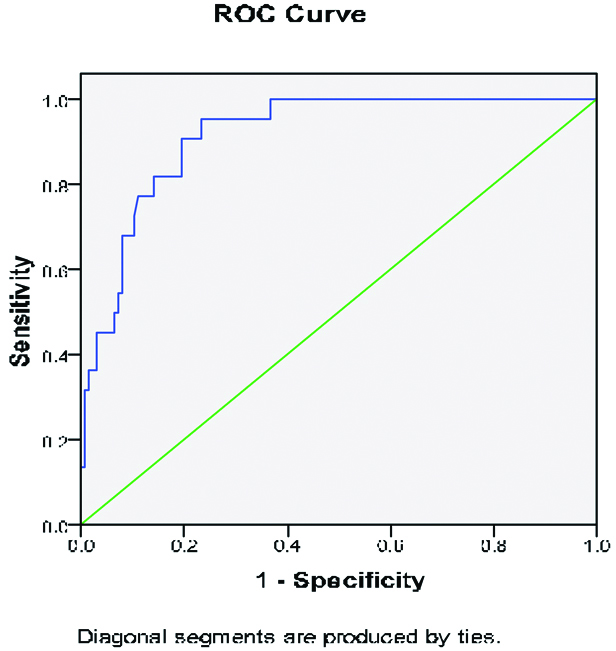
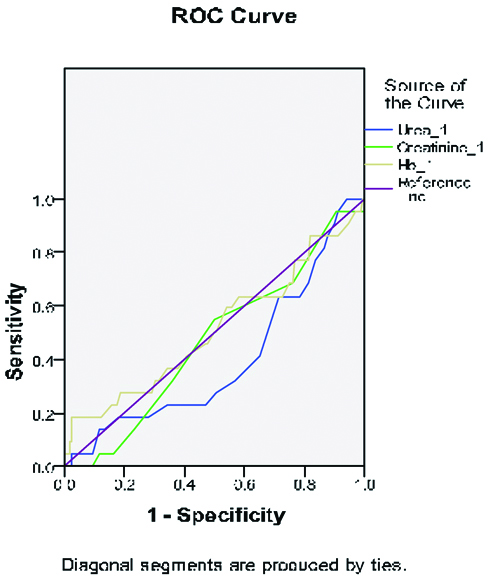
The ROC curve analysis of NT-proBNP for predicting CI-AKI is shown in ROC curve 1 [Table/Fig-6]. A cut off value of NT-proBNP of ≥2320 pg/mL as measured on admission has 90.9% sensitivity and 81.5% specificity in predicting CI-AKI. The ROC curve analysis of urea, creatinine and Hb levels at presentation is shown in ROC curve 2 [Table/Fig-7]. We did not find them to be predictive of CI-AKI in the studied patients.
The receiver operating characteristic (ROC) curve analysis for serum NT-proBNP levels in predicting of postprocedural development of CI-AKI.
 |
| Area Under the Curve |
| Test Result Variable(s): NTproBNP |
| Area | Std. Errora | Asymptotic Sig.b | Asymptotic 95% Confidence Interval |
| Lower Bound | Upper Bound |
| 0.917 | 0.025 | 0.000 | 0.868 | 0.966 |
The ROC shows that the curve intersected the sensitivity line at, <2, signifying about 95% sensitivity and area under the curve was 0.917. The ROC therefore suggests that serum NT-pro BNP levels predict post-procedural development of CI-AKI.
The test result variable(s): NTproBNP has at least one tie between the positive actual state group and the negative actual state group. Statistics may be biased.
a. Under the nonparametric assumption
b. Null hypothesis: true area = 0.5
The receiver operating characteristic (ROC) curve analysis for urea, creatinine and Hb in predicting of post-procedural development of CI-AKI.
The test result variable(s): Urea_1, Creatinine_1, Hb_1 has at least one tie between the positive actual state group and the negative actual state group. Statistics may be biased.
a. Under the nonparametric assumption
b. Null hypothesis: true area = 0.5
Discussion
The CI-AKI is an unwelcome complication after PCI which leads to unnecessarily prolonging hospital stay, may increase the risk of repeat revascularisation, and has been associated with a high mortality rate [8]. McCullough PA and Soman SS, showed that patients who require dialysis after developing CI-AKI have 36% in hospital mortality and 19% two-year mortality rate [9]. Development of CI-AKI is a marker for poor prognosis after revascularisation for ACS [10].
Patients with pre-existing renal impairment, diabetes, anaemia, and heart failure are at higher risk for developing CI-AKI. Patients with pre-existing renal impairment and cardiogenic shock patients were excluded in the present study. The use of a higher volume of contrast media and lack of attention to maintaining intravascular volume prior and during the procedure also predispose to the development of CI-AKI [8,11]. Hydration started before procedure with normal saline at 60-75 mL/hour total of 1-1.2 litres and faster rates in cases of inferior wall myocardial infarction.
The clinical and prognostic relevance of CI-AKI in STEMI patients undergoing primary PCI was first reported by Sadeghi HM et al., in a sub-study of the CADILLAC trial. They reported an incidence of 4.6% among patients undergoing primary PCI [12]. Patel UD et al., showed that inpatients undergoing cardiac surgery, the risk of postoperative AKI correlated with preoperative BNP levels [13]. Agkool O et al., demonstrated that in STEMI patients BNP levels play an important role in identifying patients at risk for AKI from any cause, not only CI-AKI [14].
The NT-proBNP is released from ventricles in response to increased ventricular wall stress and also from neurohormonal activation [15,16]. Myocardial infarction cause acute rise in Left Ventricular End Diastolic Pressure (LVEDP) which stimulate its release [17]. NT-proBNP causes depression of myocardial contractility by inhibiting the sarcoplasmic reticulum Ca2+ATPase and increasing matrix metalloproteinases [18]. NT-proBNP is a strong predictor of both short and long-term mortality in ACS patients [19]. Various pathophysiologic mechanisms including Intra-renal vasoconstriction, medullary hypoxia, oxidative stress, endothelial dysfunction, and direct tubular epithelial cell injury by contrast media have been proposed for CI-AKI. Previous studies have demonstrated that pre-procedural predictors of CI-AKI include age, baseline renal function, contrast volume and ejection fraction [20]. In the present study, we did not observe a predictive correlation (as evidenced by the ROC) between baseline serum creatinine and urea levels and CI-AKI. However, we did find a positive correlation between LVEF< 40% and CI-AKI. Patients in the CI-AKI group in the present study were also found to be significantly older than patients who did not develop CI-AKI; this was in agreement with findings from prior studies. We did not analyse the volume of contrast used. Even though diabetes has been described as a risk factor for CI-AKI, we did not find a significant association between diabetes or hypertension and CI-AKI in the present study. We also did not find correlation between prior use of Angiotensin Converting Enzyme Inhibitor (ACEIs) or Angiotensin Receptor Blocker (ARBs) and the development of CI-AKI.
Limitation
We did not analyse the volume of contrast used, though very less patients exceeded maximum contrast volume.
Conclusion
Higher Baseline NT-proBNP levels (with a cut off value of 2620.46 pg/mL) can predict the development of CI-AKI after PTCA in patient with ACS. In the present study, baseline Urea, Creatinine, and Haemoglobin levels did not correlate with or predict the development of CI-AKI. Easily available NT-proBNP can be used as a marker for development of CI –AKI.
ACS=Acute coronary syndrome; CABG=Coronary artery bypass graft; CI-AKI=Contrast induced acute kidney injury; LVEF=Left ventricular ejection fraction; NSTE=non-ST-segment elevation; STE=ST-segment elevation; BMI=Body mass index; ACEI=Angiotensin converting enzyme inhibitor; ARB=Angiotensin receptor blocker
CK-MB-Creatine kinase-myocardial band; eGFR-Estimated glomerular filtration rate; HbA1c-Glycated haemoglobin; hs-CRP-High-sensitivity C-reactive protein; NT-proBNP-N-terminal pro-brain natriuretic peptide; inside the bracket the values denotes the lowest and the highest in the study population
HB-Haemoglobin; NT-proBNP=N-Terminal pro brain natriuretic peptide
Inside the bracket the values denotes the lowest and the highest in the study population
HB=Haemoglobin; NT-proBNP=N-Terminal pro brain natriuretic peptide
LVEF-Left ventricular ejection fraction
The ROC shows that the curve intersected the sensitivity line at, <2, signifying about 95% sensitivity and area under the curve was 0.917. The ROC therefore suggests that serum NT-pro BNP levels predict post-procedural development of CI-AKI.
The test result variable(s): NTproBNP has at least one tie between the positive actual state group and the negative actual state group. Statistics may be biased.
a. Under the nonparametric assumption
b. Null hypothesis: true area = 0.5
[1]. McCullough PA, Contrast-induced acute kidney injuryJ Am Coll Cardiol 2008 51(15):1419-28.10.1016/j.jacc.2007.12.03518402894 [Google Scholar] [CrossRef] [PubMed]
[2]. Gurm HS, Seth M, Kooiman J, Share D, A novel tool for reliable and accurate prediction of renal complications in patients undergoing percutaneous coronary interventionJ Am Coll Cardiol 2013 61(22):2242-48.10.1016/j.jacc.2013.03.02623721921 [Google Scholar] [CrossRef] [PubMed]
[3]. Jarai R, Dangas G, Huber K, Xu K, Brodie BR, Witzenbichler B, B-type natriuretic peptide and risk of contrast-induced acute kidney injury in acute ST-segment-elevation myocardial infarction: a substudy from the HORIZONS-AMI trialCirc Cardiovasc Interv 2012 5(6):813-20.10.1161/CIRCINTERVENTIONS.112.97235623192919 [Google Scholar] [CrossRef] [PubMed]
[4]. Tumlin J, Stacul F, Adam A, Becker CR, Davidson C, Lamcire N, Pathophysiology of contrast-induced nephropathyAm J Cardiol 2006 98(6):14-20.10.1016/j.amjcard.2006.01.02016949376 [Google Scholar] [CrossRef] [PubMed]
[5]. Firouzi A, Maadani M, Kiani R, Shakerian F, Sanati HR, Zahedmehr A, Intravenous magnesium sulfate: new method in prevention of contrast-induced nephropathy in primary percutaneous coronary interventionInt Urol Nephrol 2015 47(3):521-25.10.1007/s11255-014-0890-z25475196 [Google Scholar] [CrossRef] [PubMed]
[6]. Brown JR, Robb JF, Block CA, Schoolwerth AC, Kaplan AV, O’Connor GT, Does safe dosing of iodinated contrast prevent contrast-induced acute kidney injury?Circ Cardiovasc Interv 2010 3(4):346-50.10.1161/CIRCINTERVENTIONS.109.91063820587788 [Google Scholar] [CrossRef] [PubMed]
[7]. McDonald RJ, McDonald JS, Bida JP, Carter RE, Fleming CJ, Misra S, Intravenous contrast material induced nephropathy: casual or coincident phenomenon?Radiology 2013 267(1):106-18.10.1148/radiol.1212182323360742 [Google Scholar] [CrossRef] [PubMed]
[8]. Lazaros G, Tsiachris D, Tousoulis D, Patialiakas A, Dimitriadis K, Roussos D, In-hospital worsening renal function is an independent predictor of one-year mortality in patients with acute myocardial infarctionInt J Cardiol 2012 155(1):97-101.10.1016/j.ijcard.2010.10.02421078526 [Google Scholar] [CrossRef] [PubMed]
[9]. McCullough PA, Soman SS, Contrast-induced nephropathyCrit Care Clin 2005 21(2):261-80.10.1016/j.ccc.2004.12.00315781162 [Google Scholar] [CrossRef] [PubMed]
[10]. Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Wiviott SD, Short-term outcomes of acute myocardial infarction in patients with Acute Kidney Injury: a report from the national cardiovascular data registryCirculation 2012 125(3):497-504.10.1161/CIRCULATIONAHA.111.03990922179533 [Google Scholar] [CrossRef] [PubMed]
[11]. McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW, Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortalityAm J Med 1997 103(5):368-75.10.1016/S0002-9343(97)00150-2 [Google Scholar] [CrossRef]
[12]. Sadeghi HM, Stone GW, Grines CL, Mehran R, Dixon SR, Lansky AJ, Impact of renal insufficiency in patients undergoing primary angioplasty for acute myocardial infarctionCirculation 2003 108(22):2769-75.10.1161/01.CIR.0000103623.63687.2114638545 [Google Scholar] [CrossRef] [PubMed]
[13]. Patel UD, Garg AX, Krumholz HM, Shlipak MG, Coca SG, Sint K, Preoperative serum brain natriuretic peptide and risk of acute kidney injury after cardiac surgeryCirculation 2012 125(11):1347-55.10.1161/CIRCULATIONAHA.111.02968622322531 [Google Scholar] [CrossRef] [PubMed]
[14]. Akgul O, Uyarel H, Pusuroglu H, Isiksacan N, Turen S, Erturk M, High BNP level as risk factor for acute kidney injury and predictor of all-cause mortality in STEMI patientsHerz 2014 39(4):507-14.10.1007/s00059-013-3853-823797372 [Google Scholar] [CrossRef] [PubMed]
[15]. De Lemos JA, McGuire DK, Drazner MH, B-type natriuretic peptide in cardiovascular diseaseLancet 2003 362:316-22.10.1016/S0140-6736(03)13976-1 [Google Scholar] [CrossRef]
[16]. Sabatine MS, Morrow DA, de Lemos JA, Omland T, Desai MY, Tanasijevic M, Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemiaJ Am Coll Cardiol 2004 44(10):1988-95.10.1016/j.jacc.2004.07.05715542281 [Google Scholar] [CrossRef] [PubMed]
[17]. Staub D, Jonas N, Zellweger MJ, Nusbaumer C, Wild D, Pfisterer ME, Use of N-terminal pro-B type natriuretic peptide to detect myocardial ischemiaAm J Med 2005 118(11):128710.1016/j.amjmed.2005.05.02016271916 [Google Scholar] [CrossRef] [PubMed]
[18]. Kawakami R, Saito Y, Kishimoto I, Harada M, Kuwahara K, Takahashi N, Overexpression of brain natriuretic peptide facilitates neutrophil infiltration and cardiac matrix metalloproteinase-9 expression after acute myocardial infarctionCirculation 2004 110:3306-12.10.1161/01.CIR.0000147829.78357.C515545516 [Google Scholar] [CrossRef] [PubMed]
[19]. Omland T, Persson A, Ng L, O’Brien R, Karlsson T, Herlitz J, N-terminal pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromesCirculation 2002 106(23):2913-18.10.1161/01.CIR.0000041661.63285.AE12460871 [Google Scholar] [CrossRef] [PubMed]
[20]. Kurtul A, Duran M, Yarlioglues M, Murat SN, Demircelik MB, Ergun G, Association between n-terminal pro-brain natriuretic peptide levels and contrast-induced nephropathy in patients undergoing percutaneous coronary intervention for acute coronary syndromeClin Cardiol 2014 37(8):485-92.10.1002/clc.2229124805995 [Google Scholar] [CrossRef] [PubMed]