Anaemia is a condition in which the number of red blood cells (and consequently their oxygen carrying capacity) is insufficient to meet the body’s physiologic needs. Specific physiologic needs vary with a person’s age, gender, residential elevation above sea level (altitude), smoking behaviour, and different stages of pregnancy [1]. Anaemia holds global health concern, mainly precipitating in an underdeveloped and developing countries [2,3]. MS, a cluster of cardiometabolic disorders like central obesity, impaired glucose tolerance, dyslipidaemia and hypertension, one of the major public health problems has increasingly been reported from urban to rural areas in India [4-7]. Risk of MS increases with menopause and may partially explain the apparent acceleration in CVD after menopause [8]. The transition from pre-menopause to post-menopause is associated with the emergence of many features of the MS, including: 1) increased central (intra-abdominal) body fat; 2) a shift toward a more atherogenic lipid profile with increased low density lipoprotein and triglycerides levels, reduced high density lipoprotein, and small, dense low density lipoprotein particles; and 3) increased glucose and insulin levels [8]. Anaemia is often found with the presence of MS, though the pathophysiological theory is mostly unclear. Very few reports are available on the relation and occurrence of anaemia with MS [9-11].
Objective of this study was to determine the prevalence of anaemia and MS and to find out the relation between anaemia and MS including its component disorders among rural postmenopausal women of rural West Bengal, India.
Materials and Methods
This cross-sectional study was conducted on the postmenopausal women, aged 45-70 years, selected randomly from 30 villages of Singur block, the field practice area of All India Institute of Hygiene and Public Health (AIIH&PH), Hugli district, West Bengal, India, from 27th March, 2014 to 1st November, 2016. As there is no reported data available on the prevalence of anaemia among postmenopausal women residing either rural or urban community in India, we have considered the district fact sheet of National Family Health Survey 4 (2015-16), which have reported the prevalence of anaemia among nonpregnant women between 15-49 years, for the Hugli district which includes our study area, Singur, for calculation of the sample size of this study [12]. The prevalence of anaemia (<12.0 gm/dL of Hb) among 15-49 years of nonpregnant women was 65.7% in the district of Hugli, West Bengal, India [12]. So, taking anticipated population proportion (p) as 65.7%, proportion of women without MS (q) will be 34.3%.
Considering 95% Confidence level, the sample size was calculated by the following formula:
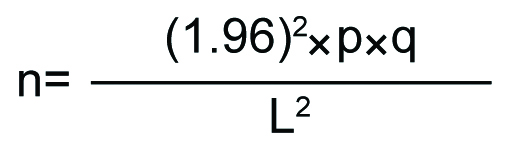
Where, L=10% proportional allowable error (i.e., 10% of p)
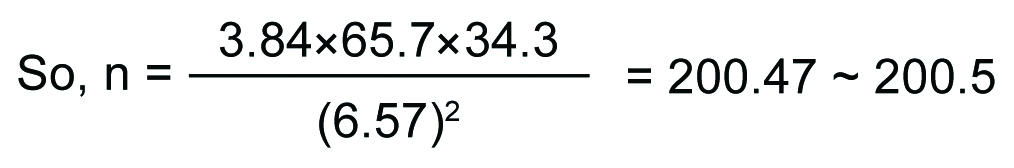
Considering the Design Effect the Sample Size will be: 200.5×2=401.
All these women were studied for determining the prevalence of MS and anaemia. Women having history of thyroid dysfunction, on hormonal replacement therapy, amenorrhea due to any pathological cause or surgery, on iron-folifer supplementation, physically or mentally challenged and non-cooperative in nature were excluded from the study. Ethical clearance was obtained from the Ethics Committee of AIIH&PH, Kolkata, India. Informed written consent was obtained prior to the study. Hb level was measured by cyanomethaemoglobin method [13]. Non anaemia, mild anaemia, moderate anaemia and severe anaemia was defined as ≥12 gm/dL, 11-11.9 gm/dL, 8-10.9 gm/dL and <8 gm/dL of Hb concentration of blood [3]. BP, WC, FBG, serum TG and HDL-C were measured using standard procedure [14-18]. Overnight fasting (10-12 hours) blood specimens were collected early in the morning from the field practice area for all biochemical estimations. MS was defined as per IDF, 2005 (for Asian-Indians) criteria [19].
Statistical Analysis
Data were put in Microsoft Excel worksheet (Microsoft, Redwoods, WA, USA) and checked for accuracy. Spearman’s correlation coefficient (rho) was calculated using SPSS software, version 20.0 (Statistical Package for the Social Sciences Inc, Chicago, IL, USA). A p-value of <0.05 was considered as statistically significant.
Results
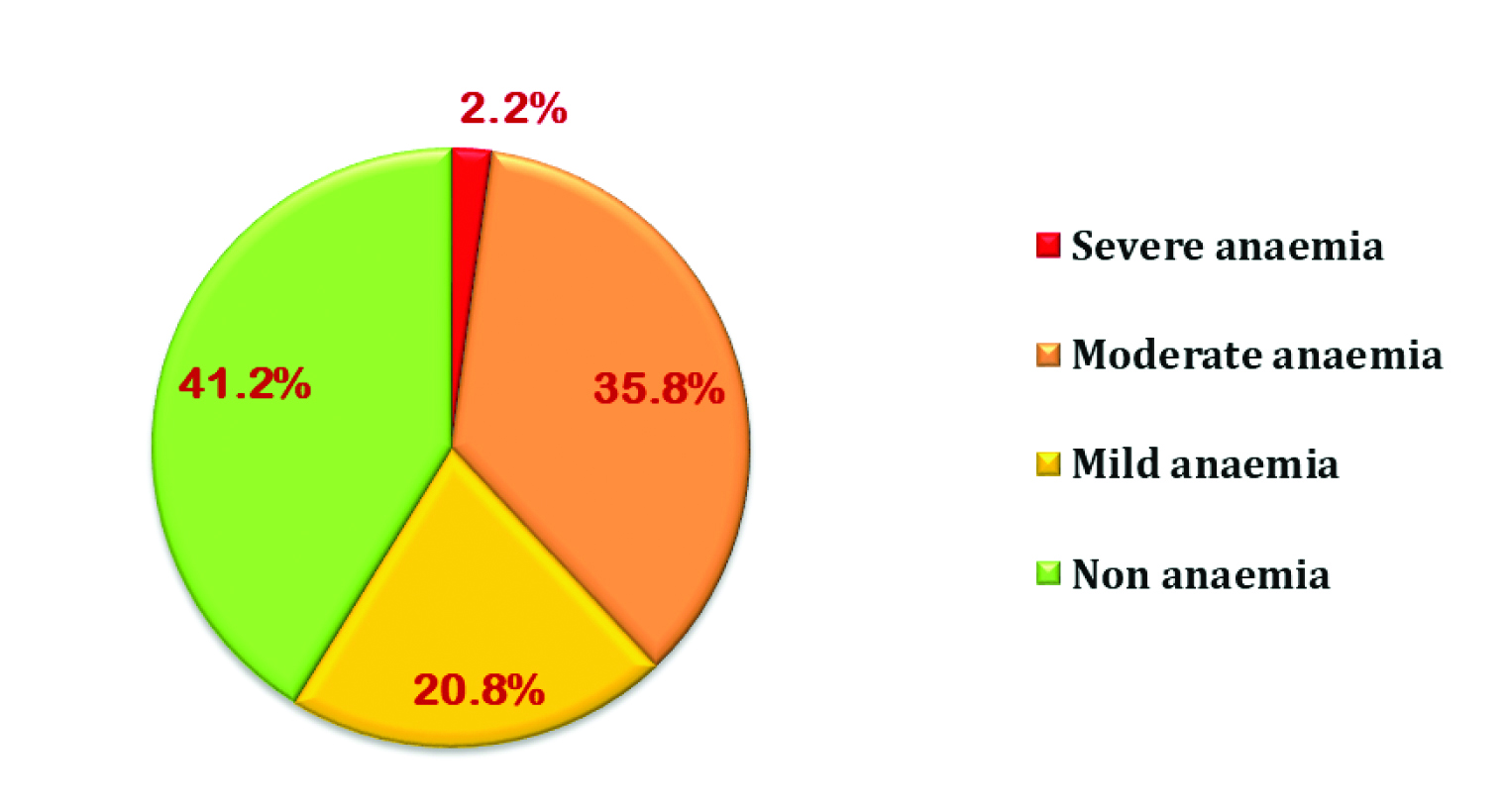
Prevalence of anaemia (Hb <12 gm/dL) among the postmenopausal women (n=509) was 58.8%. Further, mild, moderate and severe anaemia among them was observed to be 20.8%, 35.8% and 2.2%, respectively [Table/Fig-1]. Median Hb concentration was 11.5 gm/dL and Inter-Quartile Range (IQR) was 2.36 (10.5-12.85).
Distribution of the postmenopausal women according to haemoglobin status (n=509).
Prevalence of MS among the studied population (n=509) was 38.1%. Among the women having MS 54.1% was found to be anaemic. Further, among the women having WC ≥80 cm, FBG≥100 mg/dL, TG ≥150 mg/dL, HDL-C <50 mg/dL and BP ≥130/85 mmHg, 55.9%, 63.0%, 58.7%, 53.0% and 57.3% respectively, were anaemic [Table/Fig-2].
Distribution of the postmenopausal women according to haemoglobin status in relation to metabolic syndrome, waist circumference, fasting blood glucose, triglyceride, HDL cholesterol and blood pressure (N=509).
| Parameter | Haemoglobin Status (gm/dL) | Total Number (%) | Spearman’s correlation coefficient-rho (p-value) |
|---|
| Hb < 8Number (%) | Hb 8-10.9Number (%) | Hb 11-11.9Number (%) | Hb ≥12Number (%) |
|---|
| Metabolic Syndrome Present* |
| Yes | 5 (2.6) | 58 (29.9) | 42 (21.6) | 89 (45.9) | 194 (100) | Rho=0.09p=0.03 |
| No | 6 (1.9) | 124 (39.4) | 64 (20.3) | 121 (38.4) | 315 (100) |
| Waist circumference* |
| ≥80 cm | 8 (2.5) | 106 (32.9) | 66 (20.5) | 142 (44.1) | 322 (100) | Rho=0.11p=0.01 |
| <80 cm | 3 (1.6) | 76 (40.6) | 40 (21.4) | 68 (36.4) | 187 (100) |
| Fasting glucose* |
| ≥100 mg/dL | 6 (4.2) | 51 (35.7) | 33 (23.1) | 53 (37.0) | 143 (100) | Rho=-0.11p=0.01 |
| <100 mg/dL | 5 (1.4) | 131 (35.8) | 73 (19.9) | 157 (42.9) | 366 (100) |
| Triglyceride |
| ≥150 mg/dL | 2 (1.1) | 65 (35.3) | 41 (22.3) | 76 (41.3) | 184 (100) | Rho=0.02p=0.73 |
| <150 mg/dL | 9 (2.8) | 117 (36.0) | 65 (20.0) | 134 (41.2) | 325 (100) |
| HDL cholesterol* |
| <50 mg/dL | 7 (3.2) | 61 (27.9) | 48 (21.9) | 103 (47.0) | 219 (100) | Rho=-0.12p=0.01 |
| ≥50 mg/dL | 4 (1.4) | 121 (41.7) | 58 (20.0) | 107 (36.9) | 290 (100) |
| Blood Pressure** |
| ≥130/85 mmHg | 6 (1.9) | 105 (32.5) | 74 (22.9) | 138 (42.7) | 323 (100) | SBP:Rho=0.10p=0.02 |
| <130/85 mmHg | 5 (2.7) | 77 (41.4) | 32 (17.2) | 72 (38.7) | 186 (100) | DBP:Rho=-0.00p=0.95 |
*Significant (p<0.05), **Systolic BP significant (p<0.05)
Among the postmenopausal women suffering from anaemia (n=299) 35.1% were having MS. About 60.2%, 30.1%, 36.1%, 38.8% and 61.9% of anaemic women observed to have WC ≥80 cm, FBG ≥100 mg/dL, TG≥150 mg/dL, HDL-C <50 mg/dL and BP ≥130/85 mmHg. Further, 39.6%, 31.9% and 45.5% of the women suffering from mild, moderate and severe anaemia respectively were having MS. About 62.3%, 58.2% and 72.7% women with mild, moderate and severe anaemia respectively, had WC≥80 cm. Distribution of these three types of anaemia was 31.1%, 28.0% and 54.5% among the women having FBG ≥100 mg/dL. Likewise, 38.7%, 35.7% and 18.2% mild, moderate and severe anaemia was found among the women having TG≥150 mg/dL. About 45.3%, 33.5% and 63.6% mild, moderate and severe anaemia was seen in women having HDL-C <50 mg/dL. About 69.8%, 57.7% and 54.5% mild, moderate and severe anaemia was observed among the women having BP ≥130/85 mmHg [Table/Fig-3].
Distribution of anaemic postmenopausal women according to the prevalence of MS and its abnormal components (n=299).
| Anaemia StatusNo. (%) | MS presentNo. (%) | WC(≥80 cm)No. (%) | FBG(≥100 mg/dL)No. (%) | TG(≥150 mg/dL)No. (%) | HDL-C(<50 mg/dL)No. (%) | BP(≥130/85 mmHg)No. (%) |
|---|
| Mild | 106 (35.5) | 42 (39.6) | 66 (62.3) | 33 (31.1) | 41 (38.7) | 48 (45.3) | 74 (69.8) |
| Moderate | 182 (60.8) | 58 (31.9) | 106 (58.2) | 51 (28.0) | 65 (35.7) | 61 (33.5) | 105 (57.7) |
| Severe | 11(3.7) | 5 (45.5) | 8 (72.7) | 6 (54.5) | 2 (18.2) | 7 (63.6) | 6 (54.5) |
| Total | 299 (100) | 105 (35.1) | 180 (60.2) | 90 (30.1) | 108 (36.1) | 116 (38.8) | 185 (61.9) |
Statistically significant positive correlation was found between Hb level and MS (rho=0.09, p<0.05), WC (rho=0.11, p<0.05) and the systolic blood pressure (rho=0.10, p>0.05) using Spearman’s correlation test. Significant negative correlation was observed between Hb level and FBG (rho=-0.11, p<0.05) and HDL-C level (rho=-0.12, p<0.05) [Table/Fig-2].
Discussion
High prevalence of anaemia (approximately 60%), among the postmenopausal women of Singur block, West Bengal, India was observed. According to National Family Health Survey-4 (NFHS-4), more than half of the adult Indian women were anaemic and amongst them 64.8% were from rural West Bengal, which is very similar to our findings [20,21].
Our study revealed that around 40% postmenopausal women under investigation had MS. Prevalence of MS varies in India and different countries; 55% in urban Western India, 26.6% in rural north India, 64.3%in Iran, 49.8% in Brazil, 16.9% in Thailand and 29.0% in Puerto Rico [22-27].
We have found that 35.1% of the anaemic post-menopausal women were having MS and significant positive correlation was observed between these two disorders. In a study conducted in Chinese population reported among the women of 50-59 years of age the prevalence of combined anaemia and MS was highest [28].
About 60.2% of the anaemic postmenopausal women had central obesity and with increase in central obesity, prevalence of anaemia increases significantly. Other reported data corroborate our result that in all age groups anaemia was common in obesity [29].
In our study, 30.1% anaemic postmenopausal women had hyperglycaemia and decreasing Hb level was significantly correlated with increasing fasting blood glucose level (≥100 mg/dL). Other studies also documented that anaemia is commonly observed in diabetic patients and it contributes to the progression of diabetes-related complications and these are negatively associated with each other [30-34].
Around 38.8% of the studied population, suffering from anaemia, were observed to have low HDL-C. With increasing prevalence of anaemia, HDL-C level significantly decreased below 50 mg/dL. Few studies postulated that low HDL-C level is associated with occurrence of anaemia by increasing hepcidine level [35].
Besides, we have also found that around 61.9% postmenopausal women with anaemia had BP ≥130/85 mmHg. Significant positive correlation between anaemia and increased systolic blood pressure exists which indicates the co-existence of anaemia and hypertension. Previous study also reported the association of hypertension with anaemia [28].
However, no significant relationship was observed between Hb level and either diastolic blood pressure or serum TG level.
Limitation
This study observed the prevalence of total hypochromic anaemia on the basis of the Hb concentration among postmenopausal women. No attempt was taken to identify the type of anaemia, nutritional or non-nutritional, more specifically.
Conclusion
Prevalence of anaemia and MS among postmenopausal women studied in the Singur block, West Bengal, India revealed coexistence of both the health disorders. Significant correlation was observed between anaemia and MS as well as most of the components of MS in this population. Therefore, our study indicates that, in the study population, anaemia may increase the risk of developing MS among postmenopausal women or viceversa.
*Significant (p<0.05), **Systolic BP significant (p<0.05)