In the patients who are treated with insulin, the synthesis of insulin antibodies occasionally induce difficulty in glycaemic management. Insulin antibodies are now being closely examined in the context of diabetes treatment. Although, human insulin analogues are supposed to be less antigenic; however, insulin antibodies may mimic severe insulin resistance or blood glucose brittleness in some cases.
We hereby report the case of an 82-year-old male with insulin-treated Type 2 diabetes admitted to our hospital and diagnosed with diabetic ketoacidosis and acute pancreatitis. His levels of insulin antibodies and binding rate was very high. Intravenous insulin infusion and gabexate mesilate were administered. The acute pancreatitis improved but ketoacidosis re-emerged during insulin infusion. After administration of 30 mg prednisolone, infusion with insulin detemir was discontinued. On the day of discharge, his glycaemic control was good and the patient was prescribed low dose prednisolone, 8 U/day of insulin detemir, and 150 mg of miglitol. Therefore, severe immuno-insulin resistance can cause diabetic ketoacidosis and steroid might be an useful therapeutic target for insulin antibody-induced diabetic ketoacidosis.
Case Report
An 82-year-old male with Type 2 diabetes undergoing treatment with insulin was referred for general fatigue and abdominal pain after consuming alcohol, in September 2012. The patient had been diagnosed with Type 2 diabetes mellitus in 2002, and had been treated with insulin injection for the last five years (since 2007).
The patient’s treatment history of insulin injection was as follows: In 2007, at first, the patient was treated with biphasic insulin aspart 30/70 injections twice a day, before breakfast (12 U) and dinner (10 U). Level of insulin antibodies {125I-insulin binding rate=15% (normal <0.4%); insulin antibodies=4065 nU/mL} were elevated. Switching from insulin aspart to daily biphasic insulin lispro 50/50 mix injections (48 U before breakfast and 32 U before dinner) did not improve blood glucose level. The patient had no history of hypoglycaemia. In 2009, the patient was transferred to our hospital for further glycaemic control. The patient was prescribed biphasic insulin lispro 50/50 mix to biphasic insulin aspart 30 (before breakfast 30 U and dinner 8 U). The patient was a chronic alcoholic with a habit of daily consumption of alcohol and was advised to stop drinking, to which he was non complaint.
In September 2012, the patient’s anti-insulin antibody levels were significantly elevated {125I-insulin binding rate=90% (normal <0.4%); insulin antibodies >50 U/mL}. Fasting and random plasma glucose levels were constantly between 200 and 400 mg/dL. Patient’s weight was 57.5 kg and height was 156 cm (BMI, 23.6 kg/m2). The patient had no family history of diabetes. The patient’s blood pressure was 148/76 mmHg, pulse rate was 107 beats/min with a regular rhythm, and the skin turgor was reduced.
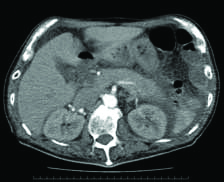
Laboratory data on admission revealed pH of blood of 7.02, partial pressure of carbon dioxide of 25.7 mmHg, oxygen of 122.1 mmHg, bicarbonate content of 6.5 mmol/L, and plasma glucose level of 728 mg/dL. The patient’s HbA1c {National Glycohemoglobin Standardization Program (NGSP)} level was 12.5%, but serum C-peptide level was detectable. Urinalysis showed ketonuria. The anti-GAD antibody test was negative. Levels of pancreatic enzymes were also elevated [Table/Fig-1]. Abdominal CT revealed pancreatic swelling and abdominal ascites [Table/Fig-2]. There were no ischaemic changes revealed by electrocardiogram. These findings were suggestive of Diabetic Ketoacidosis (DKA) and acute alcoholic pancreatitis. Intravenous infusion of saline and insulin was started.
The laboratory data on admission.
| WBC | 10810/μL | ALP | 282 U/L | IRI | 89.7μU/mL |
|---|
| RBC | 489×104/μL | γ-GTP | 36 U/L | C-peptide | 1.34 ng/mL |
| Hb | 15 g/dL | Amylase | 1172 U/L | GAD-Ab[RIA] | <0.3 U/mL |
| Ht | 45.8% | P-Amylase | 935 U/L | IA | >50 U/mL |
| Plt | 19.8×104/μL | Elastase-1 | >3000 ng/dL | IA binding rate | >90% |
| TP | 8.4 g/dL | Trypsin | 1060 ng/mL | HbA1c (NGSP) | 12.5% |
| Albumin | 4.8 g/dL | CRP | 0.23 mg/dL | pH | 7.024 |
| T-bil | 0.61 mg/dL | Na | 126 mmol/L | pCO2 | 25.7 mmHg |
| AST | 25 U/L | K | 5.6 mmol/L | pO2 | 122.1 mmHg |
| ALT | 18 U/L | Cl | 89 mmol/L | HCO3 | 6.5 mmol/L |
| LD | 154 U/L | Ca | 10.3 mg/dL | Base Excess | −23.1 mmol/L |
| CK | 52 U/L | P | 7.1 mg/dL | U-gravity | 1.025 |
| CK-MB | 12 U/L | Mg | 2.9 mg/dL | U-pH | 5.5 |
| BNP | 88.6 pg/mL | BUN | 31 mg/dL | U-protein | +/- |
| UA | 10.3 mg/dL | Cr | 0.91 mg/dL | U-glucose | 4+ |
| T-Chol | 229 mg/dL | eGFR | 60.7 mL/min/1.73 m2 | U-ketone | 3+ |
| TG | 95 mg/dL | PG | 728 mg/dL | | |
PG: Plasma glucose; IRI: Immunoreactive insulin; Ht: Hematocrit; GAD-Ab: Glutamic Acid Decarboxylase Autoantibodies; IA: Insulin antibodies; HbA1c: Glycated Haemoglobin; U: Urine
Abdominal contrast CT findings. Abdominal CT showed pancreatic swelling and abdominal ascites.
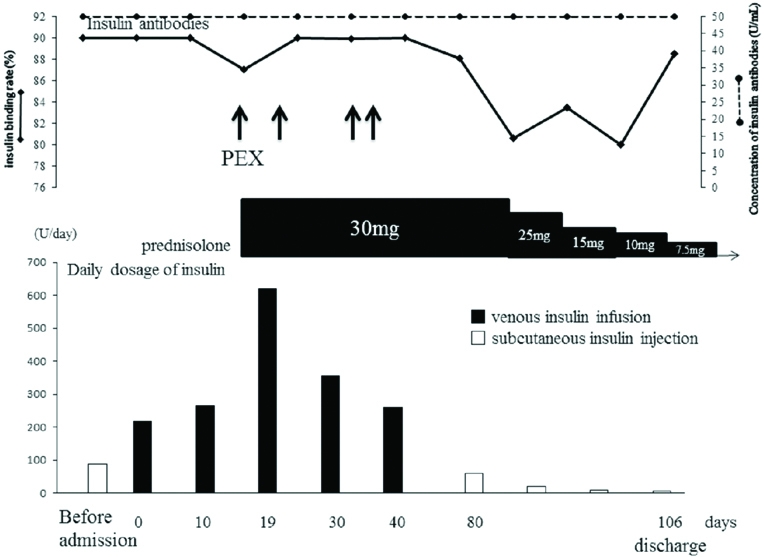
At the same time, gabexate mesilate was administered to treat acute pancreatitis. On the second hospital day, the patient’s abdominal pain decreased but acidosis worsened because of the large amount of insulin antibodies. After 10 days of gabexate mesilate treatment, the patient’s pancreatic amylase level was normalised and abdominal pain was reduced. Notably, a 200 U/day intravenous insulin infusion was needed to maintain a 200 mg/dL plasma glucose level. On the 19th hospital day, DKA recurred with a serum glucose level of 723 mg/dL, pH of 6.96, and ketonuria. The maximum intravenous insulin dosage reached up to 70 U/hour; however, this did not make a clinically-significant impact. Therefore, we performed plasmapheresis (four times) and administered 30 mg prednisolone to combat the effects of elevated levels of insulin antibodies. The titer and concentration of insulin antibodies did not change significantly. Subcutaneous injection of all insulin analogues did not improve glycaemic control and acidosis.
After starting prednisolone administration, the patient began to experience morning hypoglycaemia; therefore, the daily insulin dosages were gradually reduced. In addition, with treatment, the patient’s acidosis improved. Finally, he was discharged on the 106th hospital day with a regimen of 7.5 mg of prednisolone, 8 U/day of insulin detemir, and 150 mg of miglitol that improved glycaemic control (fasting plasma glucose level, 91 mg/dL; fasting C-peptide level, 1.0 ng/mL; and HbA1c level, 6.7%) [Table/Fig-3].
Changes of insulin and prednisolone dose during the clinical course. After prednisolone treatment, the insulin required amount decreased and discharged at 106th hospital day. The concentration of insulin antibodies did not change.
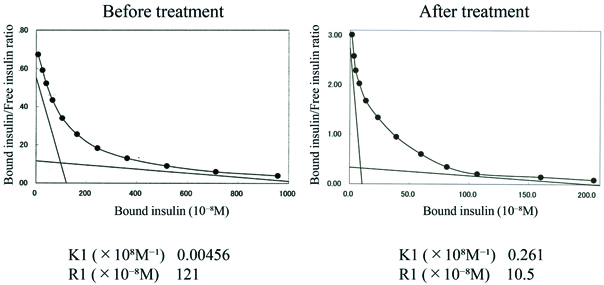
Scatchard plot analyses revealed the patient’s insulin antibody had high binding capacity and low affinity to insulin (high affinity site), which is similar to insulin autoimmune syndrome. After treatment, the binding capacity was reduced [Table/Fig-4]. The insulin receptor antibody was negative. At discharge, the binding rate of insulin antibodies was still over 90%, unaffected by the steroid treatment. After five months, we rechecked the binding rate of insulin antibodies. The rate was unchanged (over 90%).
The comparison Scatchard plot analysis of insulin antibodies before and after prednisolone treatment. Scatchard plot analyses showed the insulin antibody had high binding capacity and low affinity to insulin (high affinity site) before prednisolone treatment. After treatment, the binding capacity was reduced.
K1: affinity constant, R1: binding capacity constant of high affinity site
Discussion
Insulin antibodies to exogenous insulin often have a low capacity, high affinity property and generally do not affect glycaemic control [1-4]. However, in the present patient, insulin antibodies led to instability of glycaemic control, resulting in DKA. We suspect that the cause of the patient’s initial ketoacidosis was acute pancreatitis and severe immunogenic insulin resistance due to elevated titers of insulin antibodies and relapsed insulin-antibody-induced DKA. On the day of admission, the patient’s serum C-peptide level was 1.34 ng/mL and was not insulin dependent. Notably, his serum immunoreactive insulin was also elevated. The patient had no history of taking alpha-lipoic acid or sulfhydryl-containing drugs and no evidence of spontaneous hypoglycaemic episodes. Several case reports have proposed cessation of insulin therapy as an effective measure in the treatment of glycaemic instabilities due to insulin antibodies [5,6].
In the present patient’s clinical course, all insulin analogues, including glulisine, were administered but none improved the glycaemic levels. In addition, insulin degludec was also administered, after it was released in Japan, which was also ineffective. These results suggest that the patient’s high-titer insulin antibodies can bind all insulin analogues and human insulin, leading to severe immunological insulin resistance. In general, steroids, plasmapheresis, Double Filtration Plasmapheresis (DFPP), recombinant Insulin-like Growth-Factor-1 (IGF-1) and glucagon-like peptide-1 analogues are therapeutic choices [7-12].
We performed combination therapy of plasmapheresis four times and steroid administration to attempt rapid reduction of immunogenic insulin antibodies. We did not choose IGF-1 therapy because it is an uncommon treatment, and we did not choose DFPP because of its known transient effectiveness [8]. Plasmapheresis was not effective for glycaemic control, as indicated by the lack of change of insulin antibody binding rate (>90%) and plasma concentration (>50 U/mL). On the other hand, a continuous moderate steroid dose gradually improved the patient’s glucose level and reduced daily exogenous insulin demands. Additionally, the properties of the insulin antibodies (insulin binding capacity and affinity) were improved remarkably.
After discharge, the patient’s Scatchard analysis revealed a change in insulin antibody properties, going from an extremely high affinity and low binding capacity to a moderately high affinity and low binding capacity. It was considered that there was a possibility that steroid treatment affected the properties of the patient’s insulin antibodies.
Ishizuka T et al., reported a diabetic patient, whose insulin antibodies were 90.6%, receiving insulin (human and analogue) [1]. The insulin antibodies had low affinity and high binding capacity as revealed by Scatchard analysis. The patient was treated with DFPP and 40 mg/day prednisolone. After treatment, the binding rate of the antibodies was reduced to 30%; conversely, in the present patient binding rate was undetectably high (over 90%). In the present case, after long-term prednisolone treatment, the binding capacity constant was reduced from 121 to 10.5 (R1, ×10-8M) [Table/Fig-4]. This could contribute to the patient’s improved glycaemic control and subsequent reduction of daily insulin dosages. Although, the insulin antibody binding rate and titer were unchangeable, there is room to consider other mechanisms of glycaemic improvement from prednisolone treatment.
Insulin glargine and aspart user, long time insulin usage, multiple insulin injections and patients who have never used insulin are reported as associated factors for insulin antibody production. In the present patient, clinical history of mixed insulin aspart use and long-time (over five years) insulin usage might be associated with antibodies production [13,14]. However, there is no known decisive factor, so a future examination is required.
Conclusion
We reported a patient with high binding capacity insulin antibodies to all insulin analogues that manifested as refractory DKA. We suggest that steroid use may be key in patients with severe insulin resistance due to high-titer and high-binding insulin antibodies.
PG: Plasma glucose; IRI: Immunoreactive insulin; Ht: Hematocrit; GAD-Ab: Glutamic Acid Decarboxylase Autoantibodies; IA: Insulin antibodies; HbA1c: Glycated Haemoglobin; U: Urine
[1]. Ishizuka T, Ogawa S, Mori T, Nako K, Nakamichi T, Oka Y, Characteristics of the antibodies of two patients who developed daytime hyperglycemia and morning hypoglycemia because of insulin antibodiesDiabetes Res Clin Pract 2009 84:e21-23.10.1016/j.diabres.2009.02.00719328577 [Google Scholar] [CrossRef] [PubMed]
[2]. Fineberg SE, Kawabata TT, Finco-Kent D, Fountaine RJ, Finch GL, Krasner AS, Immunological responses to exogenous insulinEndocr Rev 2007 28:625-52.10.1210/er.2007-000217785428 [Google Scholar] [CrossRef] [PubMed]
[3]. Van Haeften TW, Clinical significance of insulin antibodies in insulin-treated diabetic patientsDiabetes Care 1989 12:641-48.10.2337/diacare.12.9.6412676431 [Google Scholar] [CrossRef] [PubMed]
[4]. Radermecker RP, Renard E, Scheen AJ, Circulating insulin antibodies: influence of continuous subcutaneous or intraperitoneal insulin infusion, and impact on glucose controlDiabetes Metab Res Rev 2009 25:491-501.10.1002/dmrr.96119496088 [Google Scholar] [CrossRef] [PubMed]
[5]. Hara K, Tobe K, Uchigata Y, Nakazono M, Yasuda K, Terauchi Y, Antibody-mediated insulin resistance treated by cessation of insulin administrationIntern Med 2000 39:143-45.10.2169/internalmedicine.39.14310732832 [Google Scholar] [CrossRef] [PubMed]
[6]. Yanai H, Adachi H, Hamasaki H, Diabetic ketosis caused by the insulin analog aspart-induced anti-insulin antibody: successful treatment with the newest insulin analog glulisineDiabetes Care 2011 34:e108doi: 10.2337/dc11-032610.2337/dc11-032621617101 [Google Scholar] [CrossRef] [PubMed]
[7]. Hirano M, Arima H, Oiso Y, Immunological insulin resistance due to insulin antibodies developed after cessation of insulin therapy in a patient with type 2 diabetesDiabetes Care 2008 31:e84doi: 10.2337/dc08-143110.2337/dc08-143118955711 [Google Scholar] [CrossRef] [PubMed]
[8]. Koyama R, Nakanishi K, Kato M, Yamashita S, Kuwahara H, Katori H, Hypoglycemia and hyperglycemia due to insulin antibodies against therapeutic human insulin: treatment with double filtration plasmapheresis and prednisoloneAm J Med Sci 2005 329:259-64.10.1097/00000441-200505000-0000715894868 [Google Scholar] [CrossRef] [PubMed]
[9]. Honda M, Kawashima Y, Kawamura H, Fujikawa H, Kikuchi K, Ohashi H, Acute liver dysfunction complicated with uncontrollable glycemia due to insulin antibody: successful treatment with glucocorticoid and lispro insulinIntern Med 2006 45:1225-29.10.2169/internalmedicine.45.600917139123 [Google Scholar] [CrossRef] [PubMed]
[10]. Matsuyoshi A, Shimoda S, Tsuruzoe K, Taketa K, Chirioka T, Sakamoto F, A case of slowly progressive type 1 diabetes with unstable glycemic control caused by unusual insulin antibody and successfully treated with steroid therapyDiabetes Res Clin Pract 2006 72:238-43.10.1016/j.diabres.2005.10.01816439034 [Google Scholar] [CrossRef] [PubMed]
[11]. Tamura Y, Kimbara Y, Funatsuki S, Mabuchi S, Kodera R, Yoshimoto A, A case of insulin antibody-induced glucose instability in an elderly woman with type 2 diabetes on hemodialysis, successfully ameliorated with liraglutideDiabetol Int 2013 4:71-75.10.1007/s13340-012-0100-0 [Google Scholar] [CrossRef]
[12]. Matsumoto Y, Yamada H, Funazaki S, Suzuki D, Kakei M, Hara K, Effect of liraglutide on type B insulin resistance syndrome and insulin allergy in type 2 diabetes: a case reportDiabetes Ther 2017 8:119110.1007/s13300-017-0291-228836180 [Google Scholar] [CrossRef] [PubMed]
[13]. Hattori N, Duhita MR, Mukai A, Matsueda M, Shimatsu A, Development of insulin antibodies and changes in titers over a long-term period in patients with type 2 diabetesClin Chim Acta 2014 433:135-38.10.1016/j.cca.2014.03.00824642342 [Google Scholar] [CrossRef] [PubMed]
[14]. Matsuyoshi A, Shimoda S, Tsuruzoe K, Taketa K, Chirioka T, Sakamoto F, A case of slowly progressive type 1 diabetes with unstable glycemic control caused by unusual insulin antibody and successfully treated with steroid therapyDiabetes Res Clin Pract 2006 72:238-43.10.1016/j.diabres.2005.10.01816439034 [Google Scholar] [CrossRef] [PubMed]