Association of Circulating Insulin-like Growth Factors and IGF Binding Protein with Early Cases of Cancer Cervix
Praveen Sablania1, Montosh Chakraborty2, Debajit Bagchi3
1 Associate Professor, Department of Biochemistry, Andaman and Nicobar Islands Institute of Medical Sciences, Port Blair, Andaman and Nicobar Islands, India.
2 Assistant Professor, Department of Biochemistry, Andaman and Nicobar Islands Institute of Medical Sciences, Port Blair, Andaman and Nicobar Islands, India.
3 Senior Resident, Department of Biochemistry, Andaman and Nicobar Islands Institute of Medical Sciences, Port Blair, Andaman and Nicobar Islands, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Praveen Sablania, Associate Professor, Department of Biochemistry, Andaman and Nicobar Islands Institute of Medical Sciences, DHS Annexe Building, Port Blair, Andaman and Nicobar Islands, India.
E-mail: sablaniapraveen@rediffmail.com
Introduction
Insulin-like Growth Factor (IGF) signaling pathway has important roles in regulating cellular proliferation, apoptosis, differentiation and transformation. IGF-axis is implicated in pathogenesis of several common cancers including cancer cervix and its pre cancer stage.
Aim
To measure the circulating levels of IGF-I, IGF-II and IGFBP-3 and evaluate their association with early stages of cancer cervix.
Materials and Methods
The present case-control study consisted of 29 histologically proven cases of early cancer cervix (FIGO Stage I and II) and 37 age matched cytologically proven normal healthy controls. The study was conducted in Department of Biochemistry, Maulana Azad Medical College, Delhi, India from May 2004-April 2007. Peripheral blood was drawn in a heparinized vial and plasma was separated by centrifugation and stored at -80°C till analysis. Concentration of IGF-I, IGF-II and IGFBP-3 was measured using commercially available ELISA kit (DRG Diagnostics) in units of ng/mL. Statistical analysis was done using SPSS statistics (V21.0) and Microsoft excel software, viz. Student-t-test for comparison of significant mean, binary logistic regression was used to measure Odds Ratios (OR). Bio-Effective Mitogen (BEM) was calculated as (IGF-I in nM+IGF-II in nM+100)–(90% IGFBP-3 in nM) and mitogenic index as BEM/10% IGFBP-3 in nM.
Results
Plasma levels of IGF-I were significantly decreased (p<0.01) while IGF-II levels were not significantly different between cases and controls (p=0.12). IGFBP-3 levels were decreased significantly in cases than controls (p<0.01). Mitogenic index was significantly increased in cases as compared to controls (p=0.01). OR for cancer cervix was calculated based on distribution of 50th percentile in control group and OR (95% CI) was 3.5 (1.04-11.73); p=0.04.
Conclusion
Circulating IGF-I, IGF-II and IGFBP-3 are in a dynamic relationship; hence, mitogenic index was educed from these factors. We found mitogenic index to be associated with cancer cervix in concordance to the cognizant role of IGFs and their binding proteins.
Biomarkers, Cervical, Neoplasia
Introduction
Cervical cancer is the second most common cancer worldwide and the commonest cancer in developing countries including India. IGF-I, IGF-II and IGFBP-3 have been implicated in the pathogenesis of various cancers including cervical cancer [1-5]. Majority of circulating IGF-I, IGF-II and IGFBP-3 are produced by liver with hepatic synthesis of IGF-I is dependent on Growth Hormone (GH) while that of IGF-II is independent of GH [6]. IGFBP-3 is a major IGF transport protein in circulation and carries 75% or more of IGFs in heterotrimeric complex that also contain Acid Labile Subunit (ALS). Approximately 90% of IGFBP-3 circulates in these complexes in a normal healthy individual [7]. IGFs exert mitogenic activity, inhibitory effect on apoptosis, effects on cell replication and differentiation as well as anabolic effects on metabolism through a heterotetrameric tyrosine kinase receptor and IGF receptor type I (IGF-IR) [8]. IGFBP-3 can have IGF dependent and IGF independent effects i.e., mitogenic activity of IGFs is inhibited by their sequestration by soluble IGFBPs while IGFBP-3 can also enhance IGFs effects by presenting and slowly releasing IGFs for interaction with their receptor [9]. IGFBP action can be modulated by IGFBP proteases as serine proteases, cathepsins, and matrix metalloproteinases [10].
Early stages of cancer cervix can be screened by pap smear but confirmation requires HPV typing, biopsy and imaging techniques. The confirmatory techniques are expensive and limited to tertiary care settings in developing countries. Thus, development of a simple biomarker, requiring less manpower and skill is the need of the hour. In this study we have tried to evaluate the association of IGF-I, IGF-II, IGFBP-3 and dynamic interplay, if any, with early stages of cervical cancer.
Materials and Methods
Sample Population
The present case-control study consisted of 29 histologically proven cases of early cancer cervix (FIGO Stage I and II) and 37 age matched cytologically proven normal healthy controls. The study was conducted in the Department of Biochemistry, Maulana Azad Medical College, Delhi, India from May 2004-April 2007 on blood samples and clinical data obtained from patients who came to attend the gynaecology outpatient department and cancer clinic at Department of Gynaecology, Maulana Azad Medical College and associated LN Hospital, New Delhi. Cases were not subjected to any surgery or treatment and they were matched with respect to age and menopausal status to controls. The study excluded women with any concurrent illness, diabetes and women on hormone replacement therapy.
Procedure
An informed consent was taken from both cases and controls, and the study was approved by Institutional Ethics Committee. Peripheral blood was drawn from antecubital vein in heparinized vial and plasma was separated by centrifugation and stored at -80°C till analysis. Concentration of IGF-I, IGF-II and IGFBP-3 was measured using commercially available ELISA kit (DRG Diagnostics) in units of ng/mL. Plasma samples were analysed in duplicate for each subject and Coefficient of Variation (CV) for duplicates were less than 10% for all variables and intra-assay and inter-assay precision for CV ranges were 5.8-6.4%, 4.1-6.9% and 4.4-7.1% for IGF-I, IGF-II and IGFBP3 respectively.
Statistical Analysis
Statistical analysis was done using SPSS statistics (V21.0) and Microsoft excel software, viz. Student t-test for comparison for significant mean, binary logistic regression and 50th percentile for estimation of OR. In all tests of significance, two sided p-value have been reported. Molar ratio between IGF-I and IGFBP-3 (MR-1) was calculated as 3.72×IGF-I/IGFBP-3. Molar ratio between IGF-II and IGFBP-3 (MR-2) was calculated as 3.82×IGF-II/IGFBP-3. Cumulative Molar Ratio (CMR) was calculated as 3.72×{IGF-I+(1.02×IGF-II)/IGFBP-3}. BEM was calculated as (IGF-I+IGF-II+100)–90% IGFBP-3 (all values in nM). Mitogenic index was calculated as BEM/10% IGFBP-3 in nM.
Results
Mean age for controls was 45.81±11.32 (range; 32-64 years) while that of cases was 50.31±12.35 (range; 35-69 years) and no statistically significant difference was observed between means of cases and controls. When cases were compared with controls, plasma levels of IGF-I were significantly decreased in cases compared to controls, while levels of IGF-II were lacking in statistical significance. IGFBP-3 levels were significantly decreased in cases compared to controls MR-1 and MR-2 were altered but these were not statistically significant. Likewise CMR and BEM were elevated in cases than controls but not associated with any statistical significance.
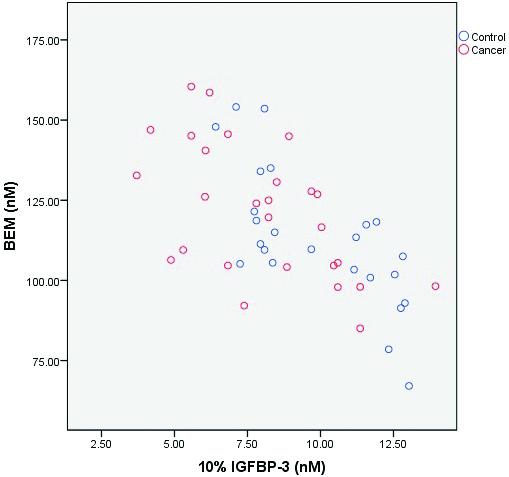
In contrast mitogenic index was increased significantly in cases compared to controls. When OR was calculated in cases, 50th percentile in control group was taken as reference, OR was statistically significant [Table/Fig-1]. Potentially free IGFBP-3 (10% of IGFBP-3) was negatively correlated with BEM (Spearman’s coefficient=-0.64; p=<0.001).
Comparison between various parameters for early cancer cases and control subjects.
| Factors*(Number) | Control | Cases | p-value |
|---|
| Mean age±SD years (N) | 45.81±11.32 (37) | 50.31±12.35 (29) | 0.13 |
| Mean IGF-I±SD ng/mL (N) | 162.64±68.65 (37) | 115.1±49.63 (29) | <0.01‡‡ |
| Mean IGF-II±SD ng/mL (N) | 623.87±105.31 (31) | 579.17±111.97 (29) | 0.12 |
| Mean IGFBP-3±SD ng/mL (N) | 2835.0±653.45 (24) | 2306.62±724.14 (27) | <0.01‡‡ |
| MR-1±SD† (N) | 0.17±0.07 (24) | 0.19±0.06 (27) | 0.29 |
| MR-2±SD‡ (N) | 0.89±0.22 (24) | 1.04±0.32 (27) | 0.07 |
| CMR±SD§ (N) | 1.07±0.26 (24) | 1.24±0.36 (27) | 0.07 |
| BEM±SDπ (N) | 113.05±21.2 (24) | 121.39±20.83 (27) | 0.16 |
| Mitogenic Index±SD** (N) | 12.41±4.78 (24) | 17.25±7.90 (27) | 0.01‡‡ |
| Mitogenic Index </=11.97 (50th percentile) | 12 | 6 | OR (95% CI)††=3.5 (1.04-11.73)p=0.04‡‡ |
| Mitogenic Index >11.97 (50th percentile) | 12 | 21 | |
*All values are calculated by using concentration of factors in ng/mL except BEM and mitogenic index which are calculated by considering nM.
†MR-1=3.72×IGF-I/IGFBP-3.
‡MR-2=3.82×IGF-II/IGFBP-3.
§CMR=3.72×(IGF-I+1.02×IGF-II)/IGFBP-3.
πBEM=(IGF-I+IGF-II+100)–90% IGFBP-3.
**Mitogenic Index=BEM/10% IGFBP-3.
††OR (95% CI)=Odds Ratio (95% Confidence Interval).
‡‡p-value significant at <0.05
Discussion
The present study attempted to evaluate the association between plasma levels of IGF-I, IGF-II and IGFBP-3 and cancer cervix. Plasma levels of IGF-I were significantly decreased in cases compared to controls (p<0.01) while levels of IGF-II were decreased in cases than controls which did not show any statistical significance (p=0.12) [Table/Fig-1,2]. In a small case-control study, Ayabe T et al., found that IGF-I levels were decreased in post-menopausal women while increased in premenopausal women and these changes were not statistically significant [11]. Serrano ML et al., reported that decrease of serum and mRNA levels of IGF-I and II levels were statistically significant [4,12] while Lee SW et al., observed statistically significant decrease in serum IGF-I levels in cancer versus control group [13]. In contrast, according to studies consisting of small sample size, Mathur SP et al., found that serum IGF-II levels were significantly increased in pre therapy cancer cervix compared to controls and significant decrease in post therapy cancer cervix compared to pre therapy levels [5,14,15]. In the present study, 70% of cancer cases were post menopausal, thus corroborating the findings of Ayabe T et al., and also consistent with the results of studies discussed so far [4,11-13]. Numerous in vitro studies have established that IGF-I exerts its mitogenic actions by several pathways that may include IGF-I/IRS-I axis, Wnt pathway, increased angiogenesis, increased production of cyclin D1 [16,17] and IGF-I promoter P1 polymorphism with risk of cervical cancer [18]. Higher circulating levels of IGF-I have been implicated in prostrate cancer, colorectal cancer, breast cancer, ovarian cancer and lung cancer [19]. In an earlier study on squamous intraepithelial lesion of cervix [20], it was found that IGF-II levels were significantly increased in pre cancer stages but it was not evident in the current study.
Comparison between IGF-I, IGF-II, IGFBP-3 (in nM) and Mitogenic index (ratio) in cancer and controls.
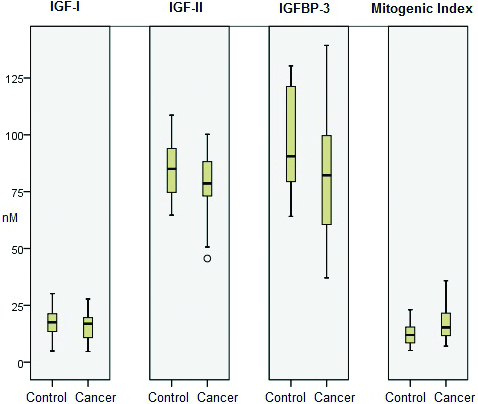
In the present study, levels of IGFBP-3 were decreased significantly in cases than controls (p<0.01) [Table/Fig-1,2]. Serrano ML et al., also described a marginally significant decreased level of IGFBP-3 in cancer cervix [4]. Similarly, Mathur SP et al., also found IGFBP-3 levels to be significantly decreased in cases than controls [15]. Another study has also reported significant decrease in mRNA levels of IGFBP-3 [12] while Sharma M et al., found both IGFBP-3 levels and its mRNA level to be significantly decreased in advanced cancer cervix [21]. Lee SW et al., demonstrated slight decrease in IGFBP-3 levels which were not statistically significant [13]. In contradiction to these findings, Huang YF et al., noticed that IGF-I and IGFBP-3 failed to predict deaths and recurrence in five-year survival study on early stage cancer cervix [19]. In addition to modulating IGF actions, IGFBP-3 may exert IGF-independent pro-apoptotic action on cellular growth through modulating TGF-β signaling, PI3-kinase activity, STAT1, MAPK/ERK, RXR-α and IGFBP-3 gene being modulated by VDR [22-27]. Increased circulating IGFBP-3 levels could reduce the risk of breast cancer, prostate and lung cancers associated with high IGF-I levels [28-30]. Conversely low IGFBP-3 levels were associated with increased risk of prostate cancer, colorectal cancer, lung cancer and breast cancer [6].
Since, IGF-I, IGF-II and IGFBP-3 have integral physiological relationship, MR-1 and MR-2 have been traditionally used and these ratios are considered to be appropriate indices of IGF-I, II and IGFBP-3 activities than activities of any of them taken separately. In the present study, it was found that MR-1 and MR-2 were slightly increased which was statistically insignificant in cases vs. controls (p=0.29 and p=0.07, respectively). CMR was found to be increased but insignificant (p=0.07) [Table/Fig-1]. In a study by Lee SW et al., IGF-I:IGFBP-3 molar ratio was found to be increased insignificantly [13] while in another study Serrano ML et al., showed statistically significant decrease in molar ratio in cases compared to controls [4]. The conflicting results of molar ratios in studies discussed so far [13,14,20] might be explained by observing dynamic relationship between IGF-I, IGF-II and IGFBP-3. IGF-I and IGF-II bind to IGF-I R while IGFBP-3 binds to IGF-I and IGF-II and thus restricting their access to IGF-I R. IGFBP-3 is also known to inhibit growth which is independent of binding to IGFs [8,9].
The findings of the present study are suggesting a concept of BEM which is based on the fact that 90% of circulating IGFBP-3 is bound to IGFs in a heterotrimeric complex that also contains ALS [8]. Thus, molar levels of IGFBP-3 were substracted from IGF-I and IGF-II viz., BEM=(IGF-I in nM+IGF-II in nM+100)–(90% IGFBP-3 in nM). The number 100 was included to expunge the possibility of any overall negative value of BEM. Thus, BEM represents the fraction of IGFs which is available to bind to other IGFBPs or occur in free form. We correlated BEM with potentially unbound IGFBP-3 (10% IGFBP-3) and found that there was an inverse correlation between them which was statistically highly significant (p<0.001) [Table/Fig-3]. We have tried to formulate a ratio between BEM and potentially unbound IGFBP-3 (10% IGFBP-3 in nM) and designated it as mitogenic index. Mitogenic index was significantly increased in cancer cervix compared to controls (p=0.01). OR for cancer cervix was calculated based on distribution of 50th percentile in control group. Around 77.8% of cases were in higher percentile (>50th percentile) as against 50% in control and OR (95% CI) was 3.5 (1.04-11.73); p=0.04 [Table/Fig-1,2].
Correlation between bio-effective mitogen and IGFBP-3.
Limitation
Since, the present study had limited sample size, more exhaustive case-control studies are required for further evaluating this index in either diagnosis or monitoring of therapy.
Conclusion
IGFs, IGFBP-3 and their molar ratios did not seem to be associated with cancer cervix in concordance to the cognizant role of IGFs and their binding proteins. In contrast, mitogenic index, educed from these factors, was found to be coherently associated with cancer cervix.
Funding: This study was supported by postgraduate research fund of Maulana Azad Medical College, Delhi, India.
*All values are calculated by using concentration of factors in ng/mL except BEM and mitogenic index which are calculated by considering nM.
†MR-1=3.72×IGF-I/IGFBP-3.
‡MR-2=3.82×IGF-II/IGFBP-3.
§CMR=3.72×(IGF-I+1.02×IGF-II)/IGFBP-3.
πBEM=(IGF-I+IGF-II+100)–90% IGFBP-3.
**Mitogenic Index=BEM/10% IGFBP-3.
††OR (95% CI)=Odds Ratio (95% Confidence Interval).
‡‡p-value significant at <0.05
[1]. Nandakumar A, Ramnath T, Chaturvedi M, The magnitude of cancer cervix in IndiaIndian J Med Res 2009 130(3):219-21. [Google Scholar]
[2]. Grimberg A, Cohen P, Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesisJ Cell Physiol 2000 183(1):1-9.10.1002/(SICI)1097-4652(200004)183:1<1::AID-JCP1>3.0.CO;2-J [Google Scholar] [CrossRef]
[3]. Wu X, Luna GT, Zhao H, Phatak D, Spitz MR, Follen M, Serum levels of insulin like growth factor I and risk of squamous intraepithelial lesions of the cervixClin Cancer Res 2003 9:3356-61. [Google Scholar]
[4]. Serrano ML, Romero A, Cendales R, Sanchez GM, Bravo MM, Serum level of insulin like growth factor-I and -II and insulin like growth factor binding protein 3 in women with squamous intraepithelial lesions and cervical cancerBiomedica 2006 26(2):258-68.10.7705/biomedica.v26i2.141516925098 [Google Scholar] [CrossRef] [PubMed]
[5]. Mathur SP, Mathur RS, Young RC, Cervical epidermal growth factor receptor and serum insulin like growth factor II levels are potential markers for cervical cancerAm J Reprd Immunol 2000 44(4):222-30.10.1111/j.8755-8920.2000.440406.x11076094 [Google Scholar] [CrossRef] [PubMed]
[6]. Jogie-Brahim S, Feldman D, Oh Y, Unraveling insulin like growth factor binding protein-3 actions in human diseaseEndocr Rev 2009 30(5):417-37.10.1210/er.2008-002819477944 [Google Scholar] [CrossRef] [PubMed]
[7]. Baxter RC, Meka S, Firth SM, Molecular distribution of IGF-binding protein-5 in human serumJ Clin Endocrinol Metab 2002 87:271-76.10.1210/jcem.87.1.815111788658 [Google Scholar] [CrossRef] [PubMed]
[8]. Firth SM, Baxter RC, Cellular actions of the insulin-like growth factor binding proteinsEndocr Rev 2002 23(6):824-54.10.1210/er.2001-003312466191 [Google Scholar] [CrossRef] [PubMed]
[9]. Baxter RC, Nuclear actions of insulin-like growth factor binding protein-3Gene 2015 569(1):7-13.10.1016/j.gene.2015.06.02826074086 [Google Scholar] [CrossRef] [PubMed]
[10]. Wetterau LA, Moore MG, Lee KW, Shim ML, Cohen P, Novel aspects of the insulin-like growth factor binding proteinsMol Genet Metab 1999 68:161-81.10.1006/mgme.1999.292010527667 [Google Scholar] [CrossRef] [PubMed]
[11]. Ayabe T, Tsutsumi O, Sakai H, Yoshikawa H, Yang T, Kurimoto F, Increased circulating levels of insulin-like growth factor-I and decreased circulating levels of insulin-like growth factor binding protein-1 in postmenopausal women with endometrial cancerEndocr J 1997 44(3):419-24.10.1507/endocrj.44.4199279518 [Google Scholar] [CrossRef] [PubMed]
[12]. Serrano ML, Sanchez-Gomez M, Bravo MM, Insulin-like growth factor system gene expression in cervical scrapes from women with squamous intraepithelial lesions and cervical cancerGrowth Horm IGF Res 2007 17(6):492-99.10.1016/j.ghir.2007.07.00117709267 [Google Scholar] [CrossRef] [PubMed]
[13]. Lee SW, Lee YS, Lee SR, Ju W, Kim SC, Plasma levels of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 in women with cervical neoplasiaJ Gynecol Oncol 2010 21(3):174-80.10.3802/jgo.2010.21.3.17420922140 [Google Scholar] [CrossRef] [PubMed]
[14]. Mathur SP, Mathur RS, Underwood PB, Kohler MF, Creasman WT, Circulating levels of insulin-like growth factor-II and IGF binding protein 3 in cervical cancerGynecol Oncol 2003 91(3):486-93.10.1016/j.ygyno.2003.08.02314675666 [Google Scholar] [CrossRef] [PubMed]
[15]. Mathur SP, Mathur RS, Creasman WT, Underwood PB, Kohler M, Early non-invasive diagnosis of cervical cancer: beyond Pap smears and human papilloma virus (HPV) testingCancer Biomark 2005 1(2-3):183-91.10.3233/CBM-2005-12-30617192039 [Google Scholar] [CrossRef] [PubMed]
[16]. Akagi Y, Lie W, Zebrowski B, Xie K, Ellis LM, Regulation of vascular endothelial growth factor expression in human colon cancer by insulin like growth factor ICancer Res 1998 58:4008-14. [Google Scholar]
[17]. Dofourny B, Albas J, Van Teefelen HA, Van der Burg B, Steenbergh PH, Mitogenic signaling of insulin like growth factor in MCF-7 human breast cancer cells requires phosphatidylinositol and is independent of mitogen-activated protein kinaseJ Biol Chem 1997 272:31163-71.10.1074/jbc.272.49.311639388270 [Google Scholar] [CrossRef] [PubMed]
[18]. Pacholska BJ, Jozefiak A, Nowak W, Kedzia W, Kwasniewska A, Gozdzicka JA, Association of the IGF-I promoter P1 polymorphism with risk of cervical cancerEur J Gynaecol Oncol 2011 32(4):393-98. [Google Scholar]
[19]. Huang YF, Shen MR, Hsu KF, Cheng YM, Chou CY, Clinical implications of insulin-like growth factor 1 system in early-stage cervical cancerBr J Cancer 2008 99:1096-102.10.1038/sj.bjc.660466118781172 [Google Scholar] [CrossRef] [PubMed]
[20]. Sablania P, Batra S, Saxena A, Insulin-like growth factor I receptor (IGF-IR) ligands and BMI in squamous intra-epithelial lesion (SIL) of cervixJ Clin Diag Res 2016 10(2):BC11-BC15.10.7860/JCDR/2016/17113.723427042445 [Google Scholar] [CrossRef] [PubMed]
[21]. Sharma M, Satyam A, Abishek A, Kharn R, Rajappa M, Sharma A, Molecular and circulatory expression of insulin growth factors in indian females with advanced cervical cancerAsian Pacific J Cancer Prev 2012 13(12):6475-79.10.7314/APJCP.2012.13.12.647523464477 [Google Scholar] [CrossRef] [PubMed]
[22]. Fanayan S, Firth SM, Butt AJ, Baxter RC, Growth inhibition by insulin-like growth factor binding protein-3 in T47D breast cancer cells requires transforming growth factor-β (TGF-β) and the type II TGF-β receptorJ Biol Chem 2000 275:39146-51.10.1074/jbc.M00696420010993898 [Google Scholar] [CrossRef] [PubMed]
[23]. Conover CA, Bale LK, Durham SK, Powell DR, Insulin-like growth factor (IGF) binding protein-3 potentiation of IGF action is mediated through the phosphatidylinositol-3-kinase pathway and is associated with alteration in protein kinase B/AKT sensitivityEndocrinology 2000 141:3098-103.10.1210/endo.141.9.766010965879 [Google Scholar] [CrossRef] [PubMed]
[24]. Spagnoli A, Torello M, Nagalla SR, Horton WA, Pattee P, Hwa V, Identification of STAT-1 as a molecular target of insulin-like growth factor binding protein-3 (IGFBP-3) in the process of chondrogenesisJ Biol Chem 2002 277:18860-67.10.1074/jbc.M20021820011886859 [Google Scholar] [CrossRef] [PubMed]
[25]. Martin JL, Baxter RC, Oncogenic ras causes resistance to the growth inhibitor insulin-like growth factor binding protein-3 (IGFBP-3) in breast cancer cellsJ Biol Chem 1999 274:16407-11.10.1074/jbc.274.23.1640710347201 [Google Scholar] [CrossRef] [PubMed]
[26]. Liu B, Lee HY, Weinzimer SA, Powell DR, Clifford JL, Kurie JM, Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid X receptor-alpha regulate transcriptional signaling and apoptosisJ Biol Chem 2000 275:33607-13.10.1074/jbc.M00254720010874028 [Google Scholar] [CrossRef] [PubMed]
[27]. Krishnan AV, Peehl DM, Feldman D, Inhibition of prostate cancer growth by vitamin D: regulation of target gene expressionJ Cell Biochem 2003 88:363-71.10.1002/jcb.1033412520538 [Google Scholar] [CrossRef] [PubMed]
[28]. Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Circulating concentrations of insulin-like growth factor-I and risk of breast cancerLancet 1998 351:1393-96.10.1016/S0140-6736(97)10384-1 [Google Scholar] [CrossRef]
[29]. Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Plasma insulin-like growth factor-I and prostate cancer risk: a prospective studyScience 1998 279:563-66.10.1126/science.279.5350.5639438850 [Google Scholar] [CrossRef] [PubMed]
[30]. Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X, Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysisJ Natl Cancer Inst 1999 91:151-56.10.1093/jnci/91.2.1519923856 [Google Scholar] [CrossRef] [PubMed]