Introduction
Stem cells have a remarkable capacity for self-regeneration and have the potential to originate different types of cells and tissue. There is a significant occurrence of natal teeth in newborn babies and usually the treatment consists of surgical removal.
Aim
To isolate and extensively characterise stem cells derived from human natal dental pulp. For this characterisation, proliferation capacity, ultrastructural morphological evaluation and trace elements were utilised.
Materials and Methods
The study was carried out in the oral pathology laboratory during October 2016. Cells from the pulp of two natal teeth were isolated through the explant technique and separated with a STRO-1 marker. The colony forming units, cell proliferation and cell viability after plating and the growth curve were analysed. The cells were morphologically analysed through Scanning Electron Microscopy (SEM) and the trace elements were analysed using Energy Dispersive Spectroscopy (EDS).
Results
The predominant cellular morphology, observed in the stem cells separated with the STRO-1 biological marker, was fibroblastic. The study of trace elements using EDS detected chlorine, sodium and sulfur.
Conclusion
Natal teeth extracted for medical reasons could be an opportunity for everyone to preserve stem cells, permitting their use in future experimental studies.
Introduction
Dental eruption is a normal physiological process that begins with the lower central incisors, when the child is about six-month-old. However, natal teeth appear at birth or in the first month after birth [1,2].
Natal teeth can cause complications such as discomfort during suckling, irritation, and trauma to the baby’s tongue, sublingual ulceration (Riga-Fede disease), laceration of the mother’s breast, and a risk of aspiration due to mobility [3-7]. Prolonged gingival irritation may increase the incidence of gingival fibrous dysplasia. The teeth may be hypermobile, necessitating extraction [3-7].
The discovery that there are stem cells in the dental pulp was first described by Gronthos S et al., and since then it has become one of the most widely researched areas in dentistry [8,9]. The reason for this is that dental pulp is one of the most easily accessible and minimally invasive sources of mesenchymal origin for stem cells, which can be extracted from permanent teeth such as the third molars, as well as through the exfoliated deciduous teeth [10-12]. Stem cells from human exfoliated deciduous teeth represent an easily accessible and noninvasive method of harvesting stem cells, thus these stem cells can be isolated, cultured and expanded in vitro, and through the manipulation of cell culture conditions, using induction mixtures with different components, these cells can be successfully differentiated in vitro and in vivo into odontoblasts, osteoblasts, chondrocytes, adipocytes and neural cells.
Stem cells are undifferentiated cells with a significant capacity for regeneration and tissue repair and will be of great value to science, since these cells have extremely interesting characteristics that can help fight diseases such as spinal cord injury, Alzheimer’s disease, autoimmune diseases and neurological disorders such as Parkinson’s disease [13]. Nowadays, stem cells are harvested from the most varied types of dental tissue such as adult dental pulp, deciduous tooth pulp, exfoliated teeth, periodontal ligament, dental follicle, apical papillae and alveolar bone tissue [14].
Due to root resorption of the deciduous teeth, for exfoliation to occur, it is more delicate to obtain stem cells in this period as there is less pulp tissue. On the other hand, obtaining stem cells from natal teeth can lessen this difficulty, since this tooth usually ends up being extracted and discarded after surgery. Thus, these stem cells can be isolated, cultured and expanded in vitro, and through manipulation of cell culture conditions, using induction mixtures with different components, these cells can be successfully differentiated, both in vitro and in vivo, into odontoblasts, osteoblasts, chondrocytes, adipocytes and neural cells [11,14].
Therefore, we investigated the possibility of isolating stem cells from the dental pulp of these teeth. It is of paramount importance that the dentist is fully equipped, not only to make a clinical diagnosis of natal teeth, but he must also have a knowledge of the biology of stem cells in order to clarify any queries put by the parents and to decide on the possibility of using these in the future.
Materials and Methods
For this in vitro study, the extraction and expansion of stem cells obtained from the dental pulp of two natal teeth in a four-day-old newborn [Table/Fig-1] was performed. The study was carried out in the oral pathology laboratory of the University of São Paulo, in October 2016.
Clinical aspect of the natal teeth, four-day-old newborn.
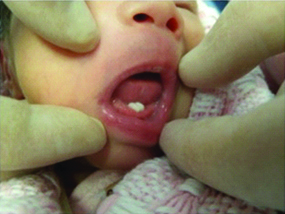
The teeth were mobile and the child’s mother asked for them to be extracted. Immediately after surgery, the teeth were placed in a test tube containing transportation matter comprising 3 mL αMEM (Minimum Essential Medium Eagle-α modification, Sigma®) plus 2% antibiotic-antimycotic solution (Gibco®), placed in a box containing ice and taken to the Oral and Maxillofacial Pathology cell harvest laboratory of the Faculty of Dentistry at the University of São Paulo, where all the harvesting procedures were performed.
Primary Culture
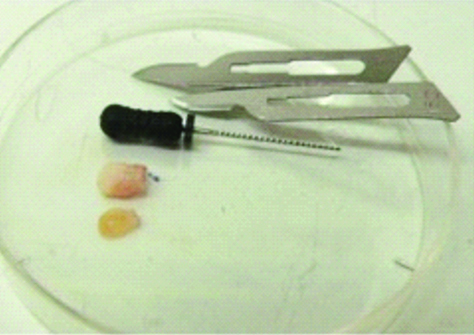
Both teeth were washed five times using a buffer solution called Phosphate-Buffered Saline (PBS) with 2% antibiotic and antimycotic (Sigma®). The dental pulp was removed using a size 15 Kerr endodontic file, as shown in [Table/Fig-2]. The explant technique was used, where the pulp tissue was fragmented and placed on a Petri dish containing harvested material, previously described by Gronthos S et al., [8]. Four days after harvesting, cells started migrating and adhering to the dish. The harvested material was replaced, according to the cell’s metabolic needs. Cell growth was monitored using an inverted phase microscope. After reaching 75% confluency, the cells were sub-harvested, the cell monolayers were washed with PBS (no calcium or magnesium, pH 7.2) and the cells were split using a solution of 2 Ml by single-cell enzymatic dissociation (trypLEE-Invitrogen) at 37°C. Cells in suspension were centrifuged at 1500 rpm for five minutes at room temperature. The cell precipitation was re-suspended in freshly harvested material and prepared for freezing. On attaining 75% confluency, the cells were detached from the dishes with the aid of the trypsin enzyme.
Removal of pulp tissue using endodontic file.
Isolation of the Stem Cells Utilising Magnetic-Activated Cell Sorting [
15]
Cells were expanded up to 1×107 so that the stem cells could be isolated using magnets. The positive selection protocol consisted of marking the cell fractions with antibodies conjugated to magnetic microspheres. Cells from the pulp tissue were then separated with a STRO-1 marker. A total of 107 cells were transferred to a 15 mL Falcon test tube, and centrifuged at 1500 rpm for 10 minutes. The floating portion was aspirated and cells were subsequently re-suspended in 80 μL of buffer solution, 10 μL of blocker with 10 μL of the antibody and incubated for an hour. After this, the cell/reagent solution was homogenised under refrigeration for 10 minutes and subsequently centrifuged at 1500 rpm for 10 minutes.
The supernatant was removed using 500 μL of buffer solution to which 10 μL of blocker and 20 μL of secondary antibody, associated with isolation microbeads, were added. The solution containing the cells was passed through a magnetic column attached to a separator.
An MTS assay was performed in order to verify the number of viable cells and if there was an increase in cell viability inhibition at different moments of the experiment: 3, 6, 9 and 12 days after plating. The test was performed on a 96-well ELISA plate. The number of plated cells was 3,500 per well, per 100 μL of matter. The cell viability test was performed with the Cell Titer 96 kit (Promega, Madison, Wisconsin, USA). A 20 μL of the Cell Titer 96® solution was placed in each well, followed by incubation for 18 minutes in a CO2 incubator at 37°C. This reaction is based on the cell conversion of the colorless tetrazolium salt (MTS) into a blue formation through dehydrogenase enzymes present in the metabolically active cells. Consequently, the wells that have more viable cells will produce more formazan salt, turning the material blue. The optical density reading, performed using an ELISA (ELX 800 Bio-TEK Instruments Inc.) device with a 490 nm filter, is proportional to the number of viable cells.
To assess the efficiency of a single-cell derived colony formation {Colony Forming Unit Fibroblast (CFU-F) assay}, 104 cells were seeded on to 10 cm2 dishes in a milliliter of the above mentioned medium. Single-cell derived colonies were defined as those units with more than 50 cells. The colonies were stained using toluidine blue solution and were counted after 7-10 days.
Analysis of Morphological Characteristics
A coverslip was placed in each well of a 12-well plate. Each tissue cell was plated in a density of 1×103 cells per well.
Cells were grown on glass coverslips (~13 mm) in 24-well plates (~110 cells per well) for 24 hours.
Afterwards, the laminates with the cultured cells were fixed with 2.5% glutaraldehyde for two hours. Three 5-minute washes were performed with 0.1 M phosphate buffer and the coverslips were left for 20 minutes in the osmium.
The process continued with further 35-minute washes with 0.1 M phosphate buffer, then the dehydration process was begun, in solutions of increasing concentrations of ethanol (30%, 50%, 70%, 90%, 95%, 100% each one for 5 minutes). After the last solution had been used, the samples were dried by immersing in Hexamethyldisilazane (HMDS, Ted Pella Inc., Redding, CA, USA) for 60 minutes and kept in a vacuum desiccator for 24 hours. Subsequently, the samples were plated with gold-palladium alloy for scanning electron microscopy (Quanta 600 FEG, FEI Company, Hillsboro, Oregon, USA), operated in secondary electron mode at a voltage of 10 kV. Each image was recorded and analysed using the Image J program.
Energy Dispersion Spectroscopy (EDS) [
17]
The quantitative information for the trace elements present in the cells, by atomic percentage or in weight, was obtained by means of the EDS technique, which is a feature of the SEM that permits a compositional analysis of the studied structure (Quanta 600 FEG, FEI Company, Hillsboro, Oregon, USA).
Results
This study has shown that it is possible to isolate stem cells from the dental pulp of natal teeth using the STRO-1 biological marker. Adherent cells were observed at the bottom of culture flasks within four days. After 7-10 days, the adherent cells proliferated, and round colonies developed in several areas of the culture. They proved to be clonogenic and proliferous. Based on a study of the cell growth curve, increasing linear expansion of the cells was observed after 3, 6, 9 and 12 days of culture in the laboratory. The most prevalent morphology of the stem cells was found to be fibroblastic. In the morphological study, using scanning electron microscopy, the cells, after being separated with STRO-1 markers, presented a morphological appearance similar to fibroblast cells [Table/Fig-3]. These same cells were analysed in back-scattered EDS mode with the same microscope, in which the most common trace elements were Chlorine (Cl), Sodium (Na) and Sulfur (S).
Morphological study: fibroblastic aspect of the stem cells.
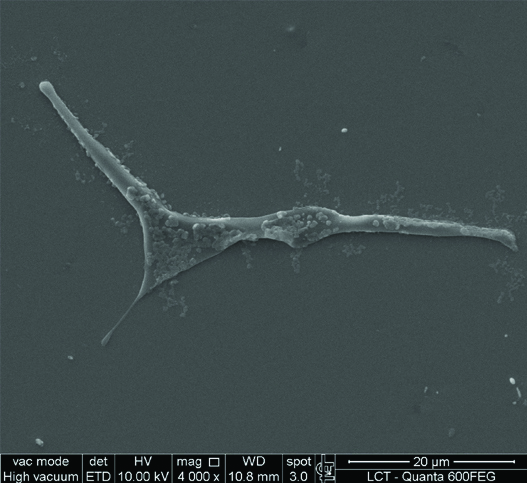
Among the trace elements, we observed the presence of the basic structure of living cell matter. The oxygen and carbon elements were discarded, as their combinations form organic molecules such as sugars (glycogen and starch). These elements represent 99% of the mass of the cells. The osmium was also discarded because it was material used in the cell preparation process for analysis under the microscope.
Discussion
Problems of babies born with birth teeth relate to incomplete or irregular formation of the tooth root, that causes the tooth to become mobile [2]. In addition, it is difficult for the mother to breastfeed because she may be injured or unable to encourage lactation because sometimes the baby may develop a pathology called Riga-Fede (a condition characterised by ulceration on the ventral surface of the tongue or on the inner surface of the lower lip) [18,19].
As far as the treatment of natal teeth is concerned, the literature unanimously opts for extraction [18], the differential being the surgical opportunity of each case. In this case report, the intervention was performed on a four-day-old patient. The possibility of finding stem cells in the dental pulp has already been evidenced in tissue engineering studies. These studies began in 2000 with Gronthos S et al., whose proposal was to use teeth as a source of stem cells [8], and these responded with a high potential for in vitro proliferation, in addition to being accessible in a less traumatic way than with bone marrow and also being an organ that is usually discarded after exfoliation, with a rich therapeutic viability and induction of tissue regeneration [20-22]. Another study aimed to isolate stem cells from exfoliated deciduous teeth and identify their phenotype and multilineage differentiation potential [11,12,14]. In vitro induction experiments have shown the potential of stem cells for osteogenic, adipogenic and neurogenic differentiation. In the morphological study using scanning electron microscopy, the cells, after being separated with STRO-1 markers, presented a morphological appearance similar to fibroblast cells. These same cells were analysed in back-scatter EDS mode using the same microscope, in which the most commonly found trace elements were noted. Using the EDS method presented, the presence of trace elements was observed. These elements represent 99% of the mass of the cells. Therefore, these elements were disregarded. We also excluded the osmium element because it is a heavy target used in the preparation of cells for microscopy analysis. Sodium, sulfur and chlorine represent the elements most frequently observed. These elements make up between 1% and 2% of the total cell mass. These elements, essential for cell function as observed in the potassium sodium pump and chlorine, are connected with the post-synaptic transmission of the cell membrane, together with Na as a mechanism for conveying cellular fluid and regulation of cell volume [23-25].
Conclusion
The cells isolated from this tissue could successfully form CFU-F units with appropriate cell surface marker STRO-1 and differentiation potential, all fulfilling the criteria per the definition of mesenchymal stem cells by the International Society of Cellular Therapy. However, more sensitive, complementary molecular tests are required as well as in vitro and in vivo differentiation to enable cell-based therapy in the future.
[1]. Malki GA, Al-Badawi EA, Dahlan MA, Natal teeth: A case report and reappraisalCase Rep Dent 2015 2015:14758010.1155/2015/14758025722895 [Google Scholar] [CrossRef] [PubMed]
[2]. Adekoya-Sofowora CA, Natal and neonatal teeth: A reviewNiger Postgrad Med J 2008 15(1):38-41. [Google Scholar]
[3]. Prabhakar AR, Ravi GR, Raju OS, Kurthukoti AJ, Shubha AB, Neonatal tooth in fraternal twins: A case reportInt J Clin Pediatr Dent 2009 2(2):40-44.10.5005/jp-journals-10005-102825206110 [Google Scholar] [CrossRef] [PubMed]
[4]. Barfiwala DR, Natal and neonatal teeth: a review of 50 casesJ Indian Soc Pedod Prev Dent 1996 14(1):21-23. [Google Scholar]
[5]. Bodenhoff J, Gorlin RJ, Natal and neonatal teeth: folklore and factPediatrics 1963 32:1087-93. [Google Scholar]
[6]. Cunha RF, Boer FA, Torriani DD, Frossard WT, Natal and neonatal teeth: review of the literaturePediatric Dentistry 2001 23(2):158-62. [Google Scholar]
[7]. Li J, Zhang YY, Wang NN, Bhandari R, Liu QQ, Riga-Fede disease in a childClin Exp Dermatol 2016 41(3):285-86.10.1111/ced.1272826307375 [Google Scholar] [CrossRef] [PubMed]
[8]. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S, Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivoProc Natl Acad Sci USA 2000 97(25):13625-30.10.1073/pnas.24030979711087820 [Google Scholar] [CrossRef] [PubMed]
[9]. Sunil PM, Manikandan R, Muthumurugan Yoithapprabhunath TR, Sivakumar M, Harvesting dental stem cells-overviewJ Pharm Bioallied Sci 2015 7(Suppl 2):S384-86.10.4103/0975-7406.16346126538883 [Google Scholar] [CrossRef] [PubMed]
[10]. Ledesma-Martinez E, Mendoza-Nunez VM, Santiago-Osorio E, Mesenchymal stem cells derived from dental pulp: A reviewStem Cells Int 2016 2016:470957210.1155/2016/470957226779263 [Google Scholar] [CrossRef] [PubMed]
[11]. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, SHED: stem cells from human exfoliated deciduous teethProc Natl Acad Sci USA 2003 100(10):5807-12.10.1073/pnas.093763510012716973 [Google Scholar] [CrossRef] [PubMed]
[12]. Sivakumar M, Dineshshankar J, Sunil PM, Nirmal RM, Sathiyajeeva J, Saravanan B, Stem cells: an insight into the therapeutic aspects from medical and dental perspectivesJ Pharm Bioallied Sci 2015 7(Suppl 2):S361-71.10.4103/0975-7406.16345326538878 [Google Scholar] [CrossRef] [PubMed]
[13]. Bojic S, Volarevic V, Ljujic B, Stojkovic M, Dental stem cells-characteristics and potentialHistol Histopathol 2014 29(6):699-706.10.1155/2014/50723424826378 [Google Scholar] [CrossRef] [PubMed]
[14]. Karaoz E, Dogan BN, Aksoy A, Gacar G, Akyuz S, Ayhan S, Isolation and in vitro characterisation of dental pulp stem cells from natal teethHistochem Cell Biol 2010 133(1):95-112.10.1007/s00418-009-0646-519816704 [Google Scholar] [CrossRef] [PubMed]
[15]. Mhaske S, Yuwanati MB, Mhaske A, Ragavendra R, Kamath K, Saawarn S, Natal and neonatal teeth: An overview of the literatureISRN Pediatr 2013 2013:95626910.1155/2013/95626924024038 [Google Scholar] [CrossRef] [PubMed]
[16]. Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, Worzella TJ, Cell viability assays. In: Sittampalam GS, Coussens NP, Brimacombe K, Grossman A, Arkin M, Auld D, et al., editorsAssay guidance manual 2004 Bethesda (MD) [Google Scholar]
[17]. Suzuki M, Morita T, Iwamoto T, Diversity of Cl(-) channelsCell Mol Life Sci 2006 63(1):12-24.10.1007/s00018-005-5336-416314923 [Google Scholar] [CrossRef] [PubMed]
[18]. Li J, Zhang YY, Wang NN, Bhandari R, Liu QQ, Riga-Fede disease in a childClin Exp Dermatol 2016 41(3):285-86.10.1111/ced.1272826307375 [Google Scholar] [CrossRef] [PubMed]
[19]. Leung AK, Robson WL, Natal teeth: A reviewJ Natl Med Assoc 2006 98(2):226-28. [Google Scholar]
[20]. Rezende KM, Bonecker M, Perez CA, Mantesso A, Synchrotron radiation X-ray micro-fluorescence: protocol to study mesenchymal stem cellsMicrosc Res Tech 2016 79(3):149-54.10.1002/jemt.2261526749077 [Google Scholar] [CrossRef] [PubMed]
[21]. Tirino V, Paino F, d’Aquino R, Desiderio V, De Rosa A, Papaccio G, Methods for the identification, characterization and banking of human DPSCs: Current strategies and perspectivesStem Cell Rev 2011 7(3):608-15.10.1007/s12015-011-9235-921318597 [Google Scholar] [CrossRef] [PubMed]
[22]. Yen AH, Sharpe PT, Stem cells and tooth tissue engineeringCell Tissue Res 2008 331(1):359-72.10.1007/s00441-007-0467-617938970 [Google Scholar] [CrossRef] [PubMed]
[23]. Zhou D, Shao L, Spitz DR, Reactive oxygen species in normal and tumor stem cellsAdv Cancer Res 2014 122:1-67.10.1016/B978-0-12-420117-0.00001-324974178 [Google Scholar] [CrossRef] [PubMed]
[24]. Williams RJ, A system’s view of the evolution of lifeJ R Soc Interface 2007 4(17):1049-70.10.1098/rsif.2007.022517439861 [Google Scholar] [CrossRef] [PubMed]
[25]. Toohey JI, Sulfhydryl dependence in primary explant hematopoietic cells. Inhibition of growth in vitro with vitamin B12 compoundsProc Natl Acad Sci USA 1975 72(1):73-77.10.1073/pnas.72.1.731054516 [Google Scholar] [CrossRef] [PubMed]