The brain is an integral part of vision. The eye collects the picture and multiple parts of the brain viz. large portions of white mater, occipital cortex, thalamus and brain stem and indirectly the cerebellum and vestibular system are involved in interpreting the collected information and then initiating an appropriate motor and/or sensory response [1-3]. Damage to any of these structures leads to visual impairment.
CVI is being identified as a leading cause of visual impairment in children both in the developed and developing world due to improved neonatal and paediatric care [4-6]. There are many causes postulated for the development of CVI like Periventricular Leucomalacia (PVL), Hypoxic Ischaemic Encephalopathy (HIE), meningitis and encephalitis involving the occipital lobes and closed head injury, hydrocephalus and brain malformations [7]. CVI was initially described as result of an insult to the retrogeniculate visual pathway and visual association areas and had no associated anterior visual pathway or ocular damage [7]. However, recent work has shown that there is a retrograde trans-synaptic degeneration of the retinal ganglion cells in children with white matter damage of immaturity resulting in associated optic nerve abnormalities [8].
Cerebral visual impairment is a diagnosis of exclusion, as many conditions like delayed visual maturation and retinal disorders mimic CVI [12]. It is a complex cognitive-perceptual dysfunction with wide variations in clinical manifestations [1], a probable reason for it to be so frequently missed by clinicians. Currently, there are no reports describing features of CVI in children with CP from the Indian subcontinent. Studies from India report the prevalence of CVI in children with CP to be between 21-28% [13,14].
At a tertiary care centre in southern India, where the present study was conducted, CVI clinic is a specialised clinic within the paediatric ophthalmology unit. The CVI clinic assesses children with CP and other neurological disorders with comprehensive neuro-ophthalmological examination and a functional visual assessment. The aim of the study is to describe the characteristics of CVI in children with CP presenting to this clinic.
Materials and Methods
This was a cross-sectional study conducted at a CVI clinic of a tertiary care multispecialty hospital in southern India from March 2011-February 2012.
All children, less than 18 years of age, referred to this clinic (by paediatricians, paediatric neurologists, child psychiatrists and ophthalmologists) for evaluation were included in the study.
The study was reviewed and approved by the Institutional Review Board and Ethics Committee.
Every child in this study underwent a complete ophthalmic examination including detailed clinical history taking, clinical observation, Best Corrected Visual Acuity (BCVA) assessment using age-appropriate visual acuity testing (preferential looking cards or optokinetic nystagmus strip, Lea grating paddles, Cardiff visual acuity cards), crowded and uncrowded visual acuity charts (Snellen’s visual acuity and Kay pictures), squint assessment, slit-lamp biomicroscopy and dilated fundus examination using indirect ophthalmoscopy. Binocular visual acuity, (using the appropriate refractive correction), was the chosen assessment due to their age and associated neurological disabilities limiting cooperation. An optometrist performed the refraction. The visual field was assessed based on the behavioural responses, reflecting the child’s ability to locate targets presented in different areas of the visual field [1].
Clinical information specifically relating to CVI was collected using a Structured Clinical Inventory [15] based on a face-to-face interview with the parents/care givers and patients who were communicative, in a non leading manner by the primary investigator (SSP). The neuropsychological (including electroencephalogram) and Magnetic Resonance Imaging (MRI) details were extracted from the case notes of the referring physician.
Cerebral palsy was classified into: 1) spastic and non spastic (dyskinetic, ataxic and mixed) depending on the gross and fine motor skills and communication skills; and 2) depending on the limbs affected as quadriplegia, diplegia and hemiplegia [16].
Features suggestive of CVI were: a) visual impairment without ocular cause; b) homonymous visual field deficits; c) difficulty handling complex visual scenes; d) apraxia of gaze; e) impaired visual guidance of upper and lower limb movement; f) impaired recognition of faces, words, shapes and objects [15]. For our study purpose, we classified CVI as absent CVI, probable CVI and definite CVI. We defined absent CVI based on history and clinical examination showing no features suggestive of CVI. Probable CVI was defined in children <1 year of age when the history revealed features suggestive of CVI but the clinical examination was inconclusive. Definite CVI was defined when there were definite features suggestive of CVI as elicited by history and clinical examination.
Statistical Analysis
Data were analysed using SSPS version 15.0. Descriptive analyses were used to summarise the profile of the study participants.
Results
A total of 341 children age ranging from 3 months-17 years, with a male preponderance (1.8:1) were examined during the period from March 2011-Feb 2012. CP was the most common diagnosis (69%, n=236). Global developmental delay or no definitive systemic diagnosis was seen in 20.7% (n=71) of the children. Definite CVI was diagnosed in 49% (n=167) of the children, 32% (n=109) were diagnosed as probable CVI and 19% (n=65) had no features suggestive of CVI at the time of examination. Definite CVI was diagnosed in 50% (n=119) of patients with CP and 49% (n=35) among those with global developmental delay or no definitive diagnosis. [Table/Fig-1] summarises the clinical diagnoses of children referred into the clinic for visual function assessment.
Distribution of clinical diagnosis and cerebral visual impairment in children attending the clinic.
| Clinical diagonosis | No CVI | Probable/Possible CVI | Definite CVI |
|---|
| Cerebral palsy | 43 | 74 | 119 |
| Autism | 12 | 6 | 7 |
| Neurodegenerative/Metabolic disorders | 0 | 1 | 2 |
| Other diagnosis (neuropsychiatric disorders) | 2 | 0 | 4 |
| No definitive diagnosis/Global developmental delay (at time of ophthalmic examination) | 8 | 28 | 35 |
Clinical Profile of Children with Cerebral Palsy [
Table/Fig-2]
Clinical profile of children with cerebral palsy.
| No CVI (n=43) | Probable CVI (n=74) | Definite CVI (n=119) |
|---|
| Median age (in months) (IQR) | 36 (24, 66) | 24 (12, 60) | 48 (24, 84) |
| Male | 29 | 45 | 78 |
| Pregnancy and Delivery |
| Uneventful mother’s antenatal period | 36 | 64 | 101 |
| Place of birth-Hospital | 43 | 72 | 117 |
| Gestational age-Term (40 wks) | 30 | 49 | 75 |
| Low birth weight (<2.5 kg) | 20 | 33 | 49 |
| Normal vaginal delivery | 21 | 42 | 61 |
| Perinatal and neonatal insults |
| Birth asphyxia (including respiratory distress) | 20 | 36 | 58 |
| Seizures | 17 | 51 | 75 |
| Jaundice | 7 | 7 | 23 |
| CNS infections | 1 | 2 | 8 |
| Others (hydrocephalus + trauma) | 2 | 2 | 2 |
| Investigations |
| Abnormal MRI | 24 (n=26) | 43 (n=47) | 61 (n=74) |
| PVL* | 11 | 22 | 22 |
| Occipital gliosis | 3 | 7 | 31 |
| Others# | 10 | 14 | 8 |
| Abnormal EEG | 24 (n=30) | 43 (n=52) | 70 (n=89) |
Causes for unavailable data were: parents could not recollect the birth history details; adopted children and investigations done elsewhere and the reports were not accessible to us
*Periventricular leukomalacia,
#hydrocephalus, delayed myelination, hypoxic ischaemic encephalopathy, atrophy, tumours
Two hundred and thirty-six children (69%) with diagnosis of CP were referred to the clinic for detailed ophthalmologic examination. CP was classified based on clinical examination as spastic quadriplegia (n=117), spastic diplegia (n=73), spastic hemiplegia (n=37) and non spastic CP (n=9). [Table/Fig-3] shows the number of CVI among the different types of CP.
Profile of cerebral visual impairment among different types of cerebral palsy.
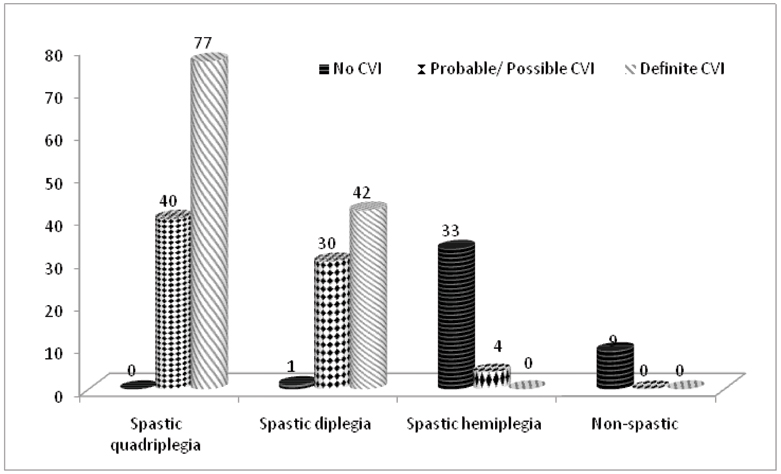
All children with spastic quadriplegia and 99% of children with spastic diplegia had features suggestive of probable CVI or definite CVI. Children with spastic hemiplegia and non spastic type of CP did not present with definite CVI.
Of the total 119 (approx. 50%) children with CP had a definite diagnosis of CVI. Age of children with CP presenting to our clinic ranged from 1-7 years. Those with definite CVI were older than 4 years of age since a good visuocognitive assessment was possible in children ≥4 years of age.
The prevalence of definite CVI in children with CP was nearly equal in boys and girls (51% vs. 49%). An uneventful maternal antenatal period was recorded in most of the mothers and was not different in children with or without CVI (85% and 84% respectively). All the children in the study were born in hospitals except two each in definite CVI and probable CVI group. Around 63% of children with definite CVI were born at term (40 weeks gestation) as compared to 70% of children without CVI. A history of neonatal asphyxia or respiratory distress (n=114) was elicited in 51% of children with definite CVI as compared to those with no CVI (17%). Seizure disorder (n=143) was more prevalent in children with definite CVI (52%) as compared to those with no CVI (12%). Of the children with definite CVI who had neuroimaging done (n=74), abnormal MRI was seen in 61 cases of which occipital gliosis were seen in 51% (n=31) of children. Abnormal findings in the EEG were reported in 79% (70 of 89 children) of children with definite CVI.
Neuro-ophthalmological Profile in Children with Cerebral Palsy
The findings of the detailed neuro-ophthalmological clinical evaluation of the children with CP (n=236) are described below. Binocular BCVA of ≤6/120 was seen in 48.7% (115 children) and binocular BCVA ≥6/19 was seen in 47 (20%) of the children. Eighty-four children (35.6%) were diagnosed with refractive error: hypermetropia-38, myopia-20, astigmatism-23, mixed astigmatism-1 and compound myopic astigmatism-2 children. Inability to correctly name basic colours (red, green, blue and yellow) was recorded in 51 (22%) children. Another 42 children had no knowledge of colours, as they had never been exposed to the experience of learning colours.
Oculomotor assessment showed strabismus in 127 (53.8%) children: exotropia in 76 (59.8%), esotropia in 43 (33.9%) children, vertical strabismus in 6 (4.7%) and combination of horizontal and vertical strabismus in 2 (1.6%) children. Nystagmus was present in 27 (11.4%) children.
Ocular fundus abnormalities were found in 68 (28.8%) children: segmental (temporal) optic disc pallor in 48 (70.6%), isolated generalised optic disc pallor in 18 (26.5%) and large optic disc cupping with pallor of the optic discs in 2 (2.9%) children.
Visual field evaluation was attempted in all the 236 children. A full examination was completed in 125 children. Among those who underwent a full visual field evaluation 24% (n=30) had no detectable visual field defect. A lower field defect 63.2% (n=79) was the commonest defect detected. A combined lower field defect and a hemifield defect was present in 16 children (12.8%). Visual field assessment was incomplete or unable to be done in 111 children (47.03%) due to the severe nature of CP and intellectual dysfunction.
Visuocognitive and Perceptual Dysfunction Profile [
Table/Fig-4]
Domains of visuocognitive and perceptual dysfunction profile in children with cerebral palsy along with radiological correlates.
| Dorsal Stream Dysfunction | Radiological features |
|---|
| Neglect | 36 (15.2%) | PVL*=4Occipital gliosis=6Others#=8Normal=1 |
| Impaired visual attention | 153 (64.8%) | PVL*=15Occipital gliosis=24Others#=39Normal=5 |
| Gaze apraxia | 36 (15.2%) | PVL*=3Occipital gliosis=6Others#=2Normal=0 |
| Foreground clutter | 60 (25.4%) | PVL*=3Occipital gliosis=15Others#=11Normal=3 |
| Background clutter | 55 (23.3%) | PVL*=2Occipital gliosis=13Others#=7Normal=3 |
| Watches television from close | 83 (35.2%) | PVL*=4Occipital gliosis=17Others#=15Normal=7 |
| Difficulty in a crowded environment | 61 (25.8%) | PVL*=7Occipital gliosis=11Others#=15Normal=3 |
| Impaired visual guidance of limbs | 68 (28.8%) | PVL*=4Occipital gliosis=17Others#=11Normal=3 |
| Ventral Stream Dysfunction | Radiological features |
| Face recognition | 111(47%) | PVL*=8Occipital gliosis=18Others#=11Normal=3 |
| Word recognition | 78 (33%) | PVL*=6Occipital gliosis=10Others#=14Normal=3 |
| Shape recognition | 61 (25.8%) | PVL*=4Occipital gliosis=10Others#=13Normal=5 |
| Object recognition | 57 (24.1%) | PVL*=2Occipital gliosis=11Others#=11Normal=3 |
| Poor memory | 51 (21.6%) | PVL*=5Occipital gliosis=8Others#=7Normal=1 |
| Orientation in familiar situations | 56 (23.7%) | PVL*=2Occipital gliosis=10Others#=10Normal=3 |
*Periventricular leukomalacia,
#hydrocephalus, delayed myelination, hypoxic ischaemic encephalopathy, atrophy, tumours.
Various aspects of visuocognitive and perceptual dysfunction (dorsal stream and the ventral stream) were assessed in all 236 children with CP. However, a complete and definitive assessment was possible only in children ≥4 years of age. Inability to recognise face and facial expressions was the commonest ventral stream dysfunction and impaired visual attention was the commonest dorsal stream dysfunction seen in children with CP.
Discussion
The brain internalises the surrounding scene through networked brain activity resulting in what we see as imagery formed in the brain [5]. Vision is therefore a complex process involving a large part of the CNS [11,17]. The lesions that cause CP like PVL, a sequel of HIE, not only affects the motor pathways but also all levels of visual pathway resulting in CVI [6]. Hence neurological disabilities like CP are often associated with various degrees of visual cognitive and perceptual dysfunction [1]. We believe that this is the first study in India that describes the wide spectrum of CVI in children with CP.
Visuocognitive dysfunction can be accurately assessed by the age of 4 years [18]. In our study, we had children as young as 3 months to as old as 17 years being referred into the clinic. About 124 of 236 (52.5%) children with CP were below the age of 4 years. It may be too early to diagnose CVI in them, as their visuocognitive functions could not be assessed. However, in the presence of perinatal insult and global developmental delay (no definitive diagnosis)/evolving CP, assessment of functional vision was important even at ages younger than 4 years and appropriate vision habilitation strategies were suggested to all the children with probable and definite CVI (n=193). Neonatal or perinatal insult like respiratory distress and seizure disorder was most commonly seen with the diagnosis of CP in the present cohort of children. In the present study 96% of the children referred for a neuro-ophthalmological evaluation had spastic CP. This is similar to other studies from India that reported a high prevalence of spastic CP (83.4-98%) [13,19]. Similar to a report in literature [20], PVL was the commonest radiological finding in the present cohort of children with CP. However, in children with CP and definite CVI, occipital gliosis was more common (51%), a finding on MRI that needs to be looked for and reported. Nearly, 49% of the children in the present study had visual acuity less than 6/120. Overall incidence of refractive error and strabismus in the present study was 36% and 54% respectively which was lesser than that reported by Fazzi E et al., [1]. Many children in the present study even in the school going age groups were unable to correctly name the basic colours. This is in contrast to the findings of Dutton GN et al., who reported that colour vision is generally preserved in children with CP and CVI [15]. This difference could be plausibly due to lack of exposure to education of children and parents.
In our experience, CVI is a common feature in children with CP. Seizure disorder was the most clinical diagnosis in children with CP and definite CVI. Radiological abnormalities were noted in 82% and an abnormal EEG in 79% of children with definite CVI. The visuocognitive dysfunction is in our study was a mixed picture of CVI with no dorsal stream or ventral stream predilection similar to previously reported in literature [21].
Limitation
In a multispecialty referral centre, children and families come from different parts of the country, with high expectations, limited time and resources and multiple consultations. This caused us to experience limitations in terms of drowsy and tired children, language, educational and socioeconomic barriers leading to an incomplete examination of the child and discussions with the family on the importance of assessment and early management of children with CP and CVI. Another limitation of the study was that Visual Evoked Potential (VEP) was not performed on any of the children. Since, this was the first attempt at evaluating our programme for these special children, we have learnt immensely and are working to modify and improve our evaluation, management and outcomes for these children to improve their quality of life.
Conclusion
In conclusion, in children who have had some perinatal or neonatal insult are at higher risk of conditions like global developmental delay including CP and learning disability. They also have features of CVI that have to be recognised. A detailed history, neuro-ophthalmological evaluation and visuocognitive assessment should be done in all children with CP irrespective of their age to enable early detection and initiation of visual habilitation strategies. Counselling of parents on the importance of visual habilitation in these special children and encouraging them to visually train them will improve the quality of life of the children.