Brucellosis has remained an emerging yet neglected zoonotic disease in humans worldwide. In developing nations like India, brucellosis remains an uncontrolled and major public health problem. The disease is mainly transmitted to humans through the ingestion of raw or unpasteurised milk and milk products contaminated with Brucella species. A wide incidence of human brucellosis in India is described in many studies ranging from 0.8% in Kashmir and 26.6% in Ludhiana [1]. In 5-10% of cases, existing infection may contribute to severe complications of the Central Nervous System (CNS) known as neurobrucellosis [2-4].
Neurobrucellosis is a rare disease and can be misdiagnosed clinically with neurological complications of other diseases of bacterial and viral origin. Despite being a rare complication of brucellosis, neurobrucellosis can be a major cause of neurological morbidity and mortality if not promptly recognised and treated. Various studies suggest that CNS involvement of brucellosis can lead to encephalitis, meningoencephalitis, radiculitis, myelitis, peripheral and cranial neuropathies, subarachnoid haemorrhage, and psychiatric manifestations [5-10].
Diagnostic criteria for neurobrucellosis are often challenging. Existing tests for diagnosis of neurobrucellosis in CSF such as culture, and molecular tests suffer from sensitivity and specificity limitation due to the paucibacillary nature of the disease. Also, such tests requires elaborate infrastructural facilities which are way beyond reach for most of the diagnostic capacities in low resource settings in India [11,12]. Development of rapid and sensitive tests that can be carried out with minimal sample volume would greatly reduce diagnostic constraints and treatment delays associated with brucellosis. Detection of Brucella specific antibodies in CSF using ELISA technique is rapid, highly sensitive, cost effective and can readily be set up in a laboratory with minimum resources [11]. Such tests can be utilised along with other diagnostic protocols for screening the incidence and confirmatory diagnosis of neurobrucellosis.
In the present study, we report utility of antibody based immunoassay protocol for diagnosis and incidence of neurobrucellosis in suspected cases with neurological complications in our hospital.
Materials and Methods
Study Design and Participant Recruitment
This was a prospective cohort study conducted on 280 patients admitted to the Neurology Department of the Central India Institute of Medical Sciences (CIIMS), Nagpur, Maharashtra, India (from the April 2015 to March 2016). Patients showing infectious/non infectious neurological disorders were referred to the Neurology Department of CIIMS from different primary and secondary health centres where more than 1000 CSF samples were withdrawn and analysed annually. Out of 280 cases, 79 patients were excluded from the study as these patients showed no exposure to neurobrucellosis risk-factors. Further 88 cases showing mixed infection other than brucellosis were ruled out from the study. Out of 113 cases, 31 patients having incomplete clinical data or incomplete demographic information or failed to provide consent for the study were excluded [Table/Fig-1]. Among the 82 selected cases, 22 patients showing non infectious CSF profile and no clinical symptoms of brucellosis were included in the study as a control group.
Flow diagram for recruited participants in the study.
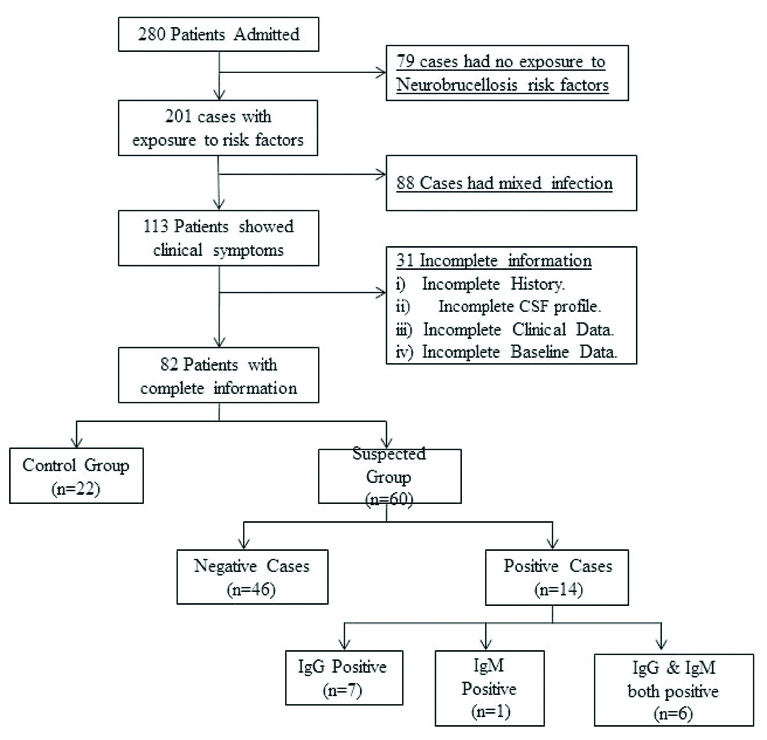
After taking the consent, total of 82 patients were recruited in the study, which included suspected (n=60) and control groups (n=22). The clinical symptoms (headache, fever, chills, ataxia, behavioural disorder, etc.,) at the time of admission were noted in a predefined format which also included demographic information like age, gender, residence, occupation, etc. The exposure to neurobrucellosis risk factors like association with infected domestic animals, intake of raw milk or milk products, occupational hazards, etc., were recorded for each patient. Radiological images of all the participants were screened for involvement of nervous system disorders like meningitis, encephalitis, brain abscess, epidural abscess, peripheral neuropathy, psychosis, etc. About 3-5 mL CSF of these patients was obtained and its parameters (sugar, protein, Total Cell Count (TCC), Gram staining, etc.,) were noted. The remaining CSF samples were divided into separate aliquots. Aliquots (1 mL) were used for, RBT, STAT and detection of Brucella specific IgG and IgM antibodies. Remaining 2 mL CSF was used for Brucella culture and identification as per method described by Pathak AD et al., [13].
Sample Collection
After taking written consent from the patients, lumbar puncture was done and 3-5 mL CSF was withdrawn for routine analysis of CSF (appearance, TCC, Differential Cell Count (DCC), protein, glucose, Gram staining, etc.) was done. All the samples were stored at 4°C until further analysis.
Rose Bengal Test and Standard Tube Agglutination Test
The RBT and STAT were performed in CSF samples of all the participants and control group. Rose bengal antigen required for RBT and plain Brucella antigen required for STAT was procured from Karnataka Veterinary, Animal and Fisheries Sciences University (KVAFSU), Hebbal, Karnataka. The test was performed as per manufacturer’s instructions. U-shaped microtitre plate was used to perform STAT. The samples were serially diluted and after incubation for 18 hour, the results were noted. Any titre in CSF showing agglutination was considered positive by STAT.
IgG and IgM Antibody Detection
Brucella specific IgG and IgM antibodies from CSF were detected by commercially available kit from NovaTec Immunodiagnostica GmbH, Germany as per manufacturer’s instructions with little modification for CSF samples. Undiluted CSF sample, ready to use positive control, negative control, cut-off and diluent blanks were added separately to the microtitre wells coated with antigen and were incubated at 37°C for 60 minutes. After incubation, enzyme conjugated anti-human IgG or IgM antibody Horseradish Peroxidase (HRP conjugated) was added and incubated for 30 minutes in the dark at room temperature. Unbound enzyme conjugated antibodies were removed by washing, followed by addition of enzyme substrate and incubated exactly for 15 minutes at room temperature in the dark. Lastly, stop solution was added and absorbance was measured at 450 nm in an ELISA reader. The colour intensity is directly proportional to the specific antibody present. The cut-off were calculated as per manufacturers instruction of kit. The samples with absorbance values higher than 10% over the cut-off control values are considered as positive. The cut-off was the mean aborbance values of the cut-off control provided by manufacturers.
Ethics Statement
The study was approved by the Institutional Ethics Committee of Central India Institute of Medical Sciences (CIIMS), Nagpur and is in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Statistical Analysis
The data were analysed using MedCalc version 10.1.2.0. The sensitivities and specificities of the assay for diagnosis of neurobrucellosis and control groups were calculated using Receiver Operating Curve (ROC). Comparison between neurobrucellosis and control groups was done by chi-square test where, p<0.05 was considered significant. The scattered plots were made using GraphPad Prism version 6.01.
Results
Out of 280 patients admitted, a total of 60 cases were finally included in the study, according to the specified inclusion criteria while 22 cases were taken as a control group. Baseline data of the study participants are mentioned in [Table/Fig-2]. Out of 60 cases,14 cases (23%) showed positivity either for IgG or IgM or both the antibodies. Of these 14 cases, there were 10 (71%) males and 4 (29%) females. About 9 (64%) patients were of age group 18-40 years. Among the positive cases, significantly more participants (78%) belonged to rural area with high exposure to animals (71%) compared to cases negative for IgG and IgM. Among positive cases, 4 (28%) were farmers, 3 (21%) were students exposed to domestic animals and family members involved in animal husbandry and agriculture, 2 (14%) were working in small scale agriculture industry while 2 (14%) were unemployed. Only one patient had history of consumption of raw milk. Pyrexia of Unknown Origin (PUO), loss of appetite and behavioural changes were major clinical manifestations significantly associated in 12 (86%) patients while headache, dizziness, vomiting, weakness and ataxia were seen in 10 (71%) patients with IgG/IgM positivity.
Comparison of baseline characteristics and clinical manifestation between participants showing IgG/IgM positivity (n=14) IgG/IgM negativity (n=46) and control group (n=22).
| Characteristics | IgG/IgM positive (n=14)(%) | IgG/IgM Negative (n=46) (%) | Control Group (n=22) (%) | p-value |
|---|
| Male | 10 (71) | 29 (63) | 15 (68) | 0.9593 |
| Female | 4 (29) | 17 (37) | 7 (32) | 0.9034 |
| Age in years |
| <18 | 2 (14) | 3 (7) | 2 (9) | 0.7093 |
| 18-40 | 9 (64) | 30 (65) | 17 (77) | 0.9025 |
| 41-60 | 1 (7) | 6 (13) | 3 (14) | 0.8477 |
| >60 | 2 (14) | 7 (15) | 0 (0) | 0.2028 |
| Residence |
| Rural | 11 (79) | 9 (20) | 15 (68) | 0.0102 |
| Urban | 3 (21) | 37 (80) | 7 (32) | 0.0324 |
| Exposure to Animals | 10 (71) | 28 (61) | 16 (73) | 0.8879 |
| Diet |
| Vegetarian | 8 (57) | 17 (37) | 13 (59) | 0.5097 |
| Non-vegetarian | 6 (43) | 29 (63) | 9 (41) | 0.5629 |
| Occupation |
| Farmers | 4 (29) | 3 (7) | 6 (27) | 0.0861 |
| Students* | 3 (21) | 10 (22) | 0 (0) | 0.1033 |
| Housewife | 3 (21) | 12 (26) | 5 (23) | 0.9489 |
| Employee | 2 (14) | 16 (35) | 9 (41) | 0.4425 |
| Unemployed | 2 (14) | 5 (11) | 2 (9) | 0.9103 |
| Clinical Manifestation |
| Headache | 10 (71) | 37 (80) | 14 (64) | 0.842 |
| Loss of appetite | 12 (86) | 21 (46) | 3 (14) | 0.0293 |
| Behavioral change | 12 (86) | 27 (59) | 5 (23) | 0.1191 |
| Vomiting | 10 (71) | 15 (33) | 4 (18) | 0.0987 |
| PUO | 12 (86) | 22 (48) | 1 (5) | 0.0049 |
| Dizziness | 10 (71) | 25 (54) | 6 (27) | 0.266 |
| Chills | 6 (43) | 16 (35) | 2 (9) | 0.1512 |
| Vision (blurred/lost) | 4 (29) | 11 (24) | 1 (5) | 0.1977 |
| Aphasia | 3 (21) | 8 (17) | 0 (0) | 0.1377 |
| Weakness | 10 (71) | 14 (30) | 5 (23) | 0.1316 |
| Neck pain/stiffness | 4 (29) | 9 (20) | 1 (5) | 0.2325 |
| Back pain | 8 (57) | 23 (50) | 4 (18) | 0.1791 |
| Ataxia | 10 (71) | 26 (57) | 2 (9) | 0.0202 |
| Hearing loss | 3 (21) | 8 (17) | 0 (0) | 0.1377 |
| Confusion/disoriented | 8 (57) | 16 (35) | 3 (14) | 0.1488 |
| Unconscious | 8 (57) | 7 (15) | 1 (5) | 0.0098 |
*students exposed to domestic animals and family members involved in animal husbandry and agriculture, PUO-Pyrexia of unknown origin
The [Table/Fig-3] shows positivity of IgG and IgM assay in the study population. Out of 60 recruited cases, 13 cases (22%) were positive for IgG with mean absorbance of 0.745 (range 1.3-0.6). Among IgM group, 7 cases (12%) were positive with mean absorbance of 0.925 (range 1.1-0.7). The 13 cases and 7 cases of positive IgG and IgM respectively, also include 6 cases (10%) which showed positivity for both IgG and IgM in CSF samples.
Percentage positivity of IgG and IgM in the suspected cases of neurobrucellosis.
| Antibodies | No. of cases (out of 60) | % positivity | Mean OD | range of OD |
|---|
| IgG positive | 13* | 22% | 0.745 | 1.3-0.6 |
| IgM positive | 7* | 12% | 0.925 | 1.1-0.7 |
*6 cases having both IgG and IgM positive
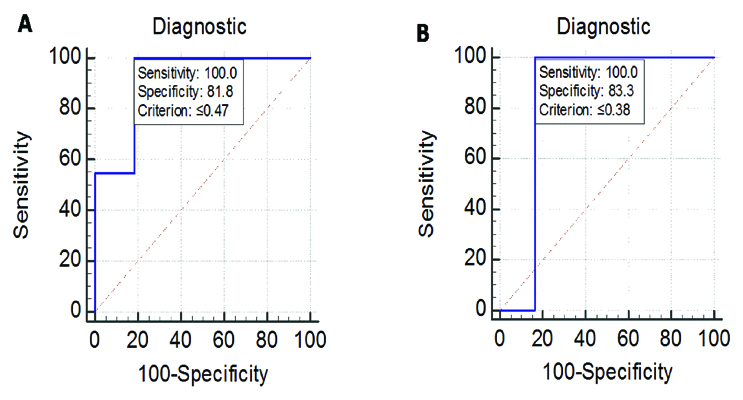
The [Table/Fig-4] shows sensitivity and specificity of IgG and IgM ELISA assay for neurobrucellosis infection. With cut-off of ≥0.47, the observed sensitivity and specificity of IgG assay was around 100% and 82%. For IgM, the sensitivity and specificity was aroud 100% and 83% with cut-off of ≥0.38.
Sensitivity and specificity of IgG/IgM in the suspected cases of neurobrucellosis.
| Sr. No | Antibody | Total no. (n) | Cut-off | Total positivity (%) | Sensitivity (%) | Specificity (%) |
|---|
| 1 | IgG | 79 | ≥0.47 | 11 (14) | 100 | 82 |
| | | | | | | |
| 2 | IgM | 79 | ≥0.38 | 6 (7.6) | 100 | 83 |
* 6 cases having both IgG and IgM positive
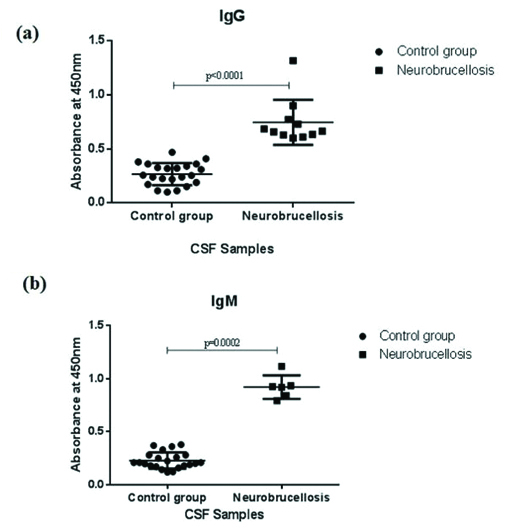
The [Table/Fig-5] shows ROC, analysis for both IgG and IgM in the CSF samples of the study participants. With a cut-off value of >=0.47, IgG ELISA method yielded sensitivity of 100% and specificity of 82% compared to IgM ELISA that showed sensitivity and specificity of 100% and 83% respectively. Both the tests yielded a fairly good sensitivity and specificity with a high percentage of concordance. The p-value (p<0.001) found was considered statistically significant. The [Table/Fig-6] shows a scatter plot of comparison of absorbance values of IgG and IgM in neurobrucellosis patients and control group. Suspected cases with neurobrucellosis were associated with significantly higher absorbance values for both IgG was (p<0.001) and IgM (p=0.002) antibodies compared to control indicating infection.
ROC (Receiver operating curve) analysis of all the samples for A) IgG, B) IgM in the study participants. The ROC plots the true positive rate (sensitivity) against the false positives (100- specificity).
Scatter plots of the absorbance values of; a) IgG and b) IgM antibodies against Brucella in CSF samples of control group and neurobrucellosis patients respectively.
p<0.05 was considered statistically significant.
The [Table/Fig-7] shows comparative positivity of RBT, STAT and Brucella culture in IgG and IgM positive cases for neurobrucellosis. About 9 (64%) out of 14 IgG/IgM positive cases showed positivity in STAT with titre values ranging from 1:16 to 1:128. Similarly, 8 (57%) out of 14 IgG/IgM positive cases showed agglutination in RBT. Among 14 cases, a total of 13 cases showed IgG antibody positivity against Brucella while seven cases was positive for IgM antibody, while 6 (43%) cases showed positivity for both IgG and IgM antibodies and were also positive by STAT and RBT. Only one IgM positive sample was found to be positive for Brucella culture after incubation for 72 hours at 37°C. The culture was positive for STAT/RBT. The positive isolate was confirmed by Gram staining as small Gram negative coccobacilli in clumps and tiny, white, non haemolytic colonies on blood agar.
Comparison of conventional RBT and STAT assay in IgG and IgM positive cases of neurobrucellosis.
| Case No. | ELISA | Agglutination tests | Brucella culture |
|---|
| IgG | IgM | RBT | STAT |
|---|
| 1 | + | - | - | - | - |
| 2 | + | - | - | + | - |
| 3 | + | - | + | - | - |
| 4 | + | + | + | + | - |
| 5 | + | - | - | - | - |
| 6 | + | + | + | + | - |
| 7 | + | + | + | + | - |
| 8 | + | + | + | + | - |
| 9 | + | - | - | + | - |
| 10 | + | - | - | - | - |
| 11 | + | + | + | + | - |
| 12 | - | + | + | + | + |
| 13 | + | - | - | - | - |
| 14 | + | + | + | + | - |
The [Table/Fig-8] shows CSF findings, radiological impression and clinical symptoms of individual participants which had positive IgG/IgM antibodies. Meningitis was a major complication observed in 7 (50%) cases whereas, hydrocephalus was found in 6 (43%) cases. Other neurological conditions like myelitis, radiculoneuritis, Peripheral Neuropathy (PNP), brain abscess, vasculitis, meningoencephalitis and sequelae were also seen in some patients. The CSF findings of all the patients showed cellularity (except two cases) ranging from 30 to 1000 cells/mL with a mean of 152.3 cells/mL. The CSF glucose levels were low with a mean of 35.15 mg/dL (except two patients). The CSF/blood glucose ratio of these cases were also lower than normal value (<60% of blood glucose level). CSF protein levels were elevated with a mean of 174.56 mg/dL.
CSF findings, radiological impressions and clinical symptoms of participants having neurobrucellosis.
| Case No. | CSF profile | Radiological Impression | Clinical symptoms |
|---|
| TLC(cells/mL) | Protein (mg/dL) | Glucose(mg/dL) | CSF/Blood glucose ratio |
|---|
| 1 | 30 | 46.78 | 28.59 | 0.37 | Meningo encephalitis | Loss of appetite, PUO, ataxia, aphasia, weakness, dizziness, back pain |
| 2 | 0 | 28.95 | 65.83 | 0.35 | Meningitis, communicating hydrocephalus | Headache, behavioural change, vomiting, blurred vision, disorientation, chills, fever |
| 3 | 30 | 44.05 | 31.45 | 0.2 | Myelitis, radiculoneuritis | Headache, behavioural change, weakness, back pain, dizziness, loss of appetite |
| 4 | >1000 | 184.9 | 24.85 | 0.31 | Meningo-encephalitis, hydrocephalus | Vomiting, weakness, neck pain, confusion, headache, PUO, ataxia, chills |
| 5 | 150 | 260 | 31.47 | 0.28 | Meningitis sequelae | Dizziness, loss of appetite, weakness, back pain, ataxia, PUO, vomiting |
| 6 | 350 | 466.1 | 55.48 | 0.53 | Epidural abscess, meningoencephalitis, psychosis | Behavioural change, disorientation, dizziness, neck stiffness, hearing loss, vomiting |
| 7 | 220 | 88.14 | 34.84 | 0.42 | Peripheral neuropathy, myelitis, radiculoneuritis | Loss of appetite, ataxia, PUO, weakness, back pain, unconscious when admitted |
| 8 | 50 | 305.2 | 46.94 | 0.45 | Brain abscess, hydrocephalus | Headache, chills, dizziness, behavioural change, vomiting, weakness |
| 9 | 150 | 121.2 | 25.7 | 0.29 | Meningitis, hydrocephalus | Loss of appetite, weakness, confusion, aphasia, behavioural change |
| 10 | 500 | 107.2 | 11.41 | 0.08 | meningitis | Rt vision lost, ataxia, back pain, PUO, weakness, headache, vomiting |
| 11 | 100 | 323.8 | 14.89 | 0.12 | Arrested hydrocephalus, meningitis, vasculitis, sequelae | Unconscious, blurred vision, behavioural change, back pain, loss of appetite |
| 12 | 100 | 196.5 | 23.49 | 0.27 | Meningitis | Headache, disorientation, vision impairment, ataxia, PUO, dizziness, aphasia, weakness |
| 13 | 0 | 50.42 | 32.81 | 0.42 | Meningo encephalitis- Brucellosis infection | Weakness, behavioral change, unconscious, disorientation PUO, neck pain |
| 14 | 300 | 220.6 | 55.48 | 0.46 | Meningitis, communicating hydrocephalus, vasculitic infarct | Vomiting, dizziness, PUO, ataxia, behavioural change, weakness, loss of appetite |
Discussion
In the present study, we report the incidence of neurobrucellosis infection and utility of CSF based antibody detection assay in suspected cases with neurological complications admitted in our hospital. Diagnosis of neurobrucellosis based on clinical symptoms and neurological complications is difficult and therefore microbial detection of Brucella in CSF is needed for confirmatory diagnosis. However, the isolation frequency of Brucella from CSF is very low due to the paucil bacillary nature of the disease, and thus the diagnosis of neurobrucellosis usually depends on indirect evidence [14-15]. Agglutination tests like STAT/RBT for Brucella specific antibodies in CSF can give false negative results for neurobrucellosis as these antibodies are also present in very low concentration in CSF [16]. Real-time Polymerase Chain Reaction (PCR) assay is sensitive [17], but it is time consuming, requires elaborate laboratory set-up and is beyond the scope of most diagnostic facilities in low-resource settings with high endemicity. On the other hand, detection of Brucella specific antibodies in CSF using ELISA technique is rapid, highly sensitive, cost effective and can readily be set up in a laboratory with minimum resources [12].
Present study showed an overall 23% incidence of neurobrucellosis in our hospital by both IgG and IgM assay which is considerabley high compared to other hospital based studies [15,18,19]. Since, 5-10% of brucellosis cases are reported to be associated with neurological involvement [2-4], present study using the existing ELISA based assay shows better diagnostic efficacy of such tests over conventinal assays for neurobrucellosis infection in suspected cases.
Headache, behavioural change, PUO, dizziness, loss of appetite, weakness, vomiting and ataxia were the major clinical symptoms observed in 90% of cases diagnosed with neurobrucellosis in present study. The present results were in agreement with other studies which have reported similar clinical and neurological manifestations associated with neurobrucellosis [2-10]. Meningitis was found in 50% of the cases, while other neurological conditions like meningoencephalitis, radiculoneuritis, brain abscess and vasculitis were observed in 14-29%. The CSF profile of recruiting participants showed a typical infectious profile with high cellularity (30-1000 cells/mL), low glucose levels (11-66 mg/dL) and high protein levels (4-466 mg/dL, except one case). Majority of cases showed neurological involvement indicating severity of disease which could be mainly due to delayed diagnosis or misdiagnosis with other bacterial diseases.
The positivity of CSF culture was around 1.3% as opposed to other studies which have shown positivity rates of 6-15% through their studies [15]. The sample was positive for IgM, RBT and STAT indicating recent infection unlike majority of cases which had earlier history of antibiotic course before admission. Five cases out of 14 ELISA positive cases, showed negative results by STAT. Similarly, six cases showed negative results by RBT which suggest false negative results by agglutination tests and a more sensitive test is required. Studies have shown low sensitivity of RBT and STAT in CSF compared to serum [19-20]. The sensitivity of CSF IgG/IgM assays in our study was 100% suggesting that such assays can be used for diagnosis even in low sample volume and minimum cost thus reducing constrains for most diagnostic settings in regions of high endemicities. Recent studies have shown that ELISA to be more reliable for diagnosis of Brucella infection compared to RBT and STAT. ELISA is capable of readily identifying individual IgM and IgG antibody to the surface antigen of Brucella bacteria, allowing for a better correlation with the clinical situation [12]. However, for accurate diagnosis in absence of CSF culture we would recommend a combitorial approach using CSF STAT/RBT along with ELISA in suspected neurobrucellosis cases to reduce false positivity and treatment delays.
In the present study showed significant occurrence of neurobrucellosis in rural areas, where the population was involved in agriculture and animal husbandry. Other urban patients might be infected through contact with mutton or consumption of incompletely sterilised milk or poorly cooked lamb and kebab. In our hospital, most of patients comprises of poor farmers, laborouers from Maharashtra and surrounding states like Chattisgargh, Madhya Pradesh. The results showed that most of positive cases of neurobrucellosis were farmers indicating the trend of brucellosis to spread toward semipastoral, pastoral, and agricultural areas. While most students and housewife positive for neurobrucellosis represented exposed cases, sharing close microenvironment with domesticated animals. The present results are in agreement with studies by Zhao S et al., in which have shown significant positivity rates of neurobrucellosis in farmers who got infected through direct contact with live cattle and sheep [21]. Among dietary habits, there was no significant association observed among positive cases, as almost equal number of positive cases consumed vegetarian and non vegetarian food. Among the age group, more number of patients in age group 18-40 years were admitted and found positive for IgG/IgM for neurobrucellosis. However, there was no significant association found with other age groups. Since increasing number of people in economically productive age groups from 18-40 are involved in occupations like farming, agriculture, there is possible likelihood of exposure and infection in such groups.
Limitation
Despite reporting high incidence of neurobrucellosis in suspected cases, present study was associated with some limitation. The major limitation of study include small sample size and low culture positivity associated with IgG/IGM positive cases. The reason for small sample size was mainly due to our strict inclusion criteria which excluded most of the suspected cases having mixed infection other than brucellosis. Since, our institute is mostly a tertiary care hospital, it is difficult to culture isolate as most suspected cases are started immediately with broad spectrum antibiotics or a referred from outside with having already taken prior antibiotic course. The CSF collection volume is another limitation since, the collected CSF undergoes wide range of tests for confirmatory diagnsosis, which hampers the positivity of culture. However, as stated elsewhere, use of combitorial approach (ELISA/STAT/RRBT) in lieu limited culture positivity should be considered diagnostic criteria for treatment initiation in suspected neurobrucellosis cases.
Conclusion
In conclusion, present study demonstrated a very high incidence of neurobrucellosis. In countries like India, with high risk-factor for brucellosis infection, proper survellience and awareness along with screening of suspected cases using antibody detection tests may greatly improve the diagnostic capacity and management associated with neurobrucellosis disease.
Financial support: The study was funded by the Indian Council of Medical Research (ICMR) (Project No. Zon/15/11/2014-EDC- II).
*students exposed to domestic animals and family members involved in animal husbandry and agriculture, PUO-Pyrexia of unknown origin
*6 cases having both IgG and IgM positive
* 6 cases having both IgG and IgM positive