In the past few years, aesthetics has gained importance along with the need for addressing biological and functional problems present in the periodontium. Situations like gingival recession around anterior teeth require one such treatment modality where both aesthetic demands and biological demands of the periodontium should be taken care of. While treating gingival recession from aesthetic and biologic point of view, the gingival type and form become very important from the beginning of treatment plan to the final stage. A variety of regenerative procedures have emerged in the past decade for the correction of gingival recession. They have shown partial or complete root coverage along with an increase in the width and height of keratinised gingiva [1].
Till now, the focus has been primarily on covering the root surface and the increasing width of the keratinised gingiva. An inadequate zone of keratinised tissue was considered as a risk factor for gingival recession [2]. Now, it has been proved that level of attachment can be maintained even in the absence of adequate zone of attached gingiva provided, that the patient maintains proper oral hygiene [3]. It has also been reported that subjects with comparatively thin gingival biotype may be more susceptible to gingival recession than subjects who belong to thick gingival biotype [4]. It was also suggested that gingival or periodontal diseases were more likely to occur in patients with a thin gingival biotype [5]. Recently, root coverage along with an increase in the thickness of gingiva has been assessed by Aroca S et al., Cardaropoli D and Cardaropoli G [6,7]. A high association between flap thickness and complete root coverage has also been reported in a meta-analysis by Hwang D and Wang HL [8]. These observations suggested that tissue biotype might be a significant factor influencing aesthetic treatment outcomes. This suggests that the main goal should not only be to attain complete root coverage but also to increase the thickness of the keratinised tissue. This might minimise the chances of relapse of gingival recession.
In this study, the human placental amnion and chorion membranes were used. These are the foetal membranes which have been used as biomaterials for scaffolds for a long time. Amnion is the inner most lining of the amniotic sac, the part of the placenta which encloses the baby through term and chorion is the next layer of the amniotic sac after the amnion. Collagen layers of amnion and chorion are rich in Type I, IV, V, VI collagen, proteoglycans, laminin and fibronectin [9]. These placental membranes are immunoprivileged tissues unlike cadaveric allograft, xenograft and alloplast barrier membranes. They possess antimicrobial and inflammation reduction properties and facilitate migration of cells by providing a protein rich matrix [10]. Natural inhibitors of matrix metalloproteinases, anti-inflammatory factors such as tissue inhibitors of metalloproteinase-1,2,3,4, interleukin-10, and interleukin-1 receptor antagonists as well as endostatin which inhibit endothelial cell proliferation, angiogenesis, and tumour growth have been isolated in human amniotic membrane [11]. Thus, based on the properties of amnion and chorion membranes this study was carried out clinically to evaluate these membranes in the treatment of gingival recession and to assess their effect on gingival biotype.
Materials and Methods
A prospective study conducted over a duration of six months was carried out on the patients visiting Department of Periodontics, MM Dental College, Mullana, Ambala, Haryana, India. Each patient was given detailed verbal and written description of the treatment with the consent to participation. Inclusion criteria included cooperative and motivated male and female patients between age groups of 20-50 years with a Miller’s Class I and II gingival recession [12]. Exclusion criteria included patients with any systemic disease, smokers, alcoholics, pregnant or nursing women, patients with any known allergy or hypersensitivity, patients with tooth mobility, patients with Class III or IV gingival recession and patients with no history of previous periodontal plastic surgery in last six months.
Twenty sites in 10 patients with gingival recession were selected randomly. The sites were divided into two groups as Group A sites which were treated with Coronally Advanced Flap (CAF) along with the placement of amnion membrane and Group B sites which were treated with CAF along with the placement of chorion membrane. The amnion and chorion membranes used in this study were obtained from Tissue Bank, Tata Memorial Hospital, Mumbai, India [Table/Fig-1].
Photograph showing Amnion (A) and Chorion (C) membranes.

Preoperative protocol: A detailed medical and dental history, periodontal assessment using clinical parameters, diagnostic casts, radiographs, clinical photographs, laboratory investigations and custom made acrylic gingival stents were done. The clinical parameters assessed at baseline before the periodontal therapy and in the follow up intervals included plaque index [13], gingival index [14], probing pocket depth (from gingival margin to the base of pocket using University of North Carolina (UNC-15) periodontal probe), relative clinical attachment level (distance from a lower/apical limit of the occlusal stent to the bottom of the pocket), position of gingival margin (CEJ to the free gingival margin using a UNC-15 periodontal probe) and gingival thickness measurement by transgingival probing (assessed manually mid-buccally in the attached gingiva, half way between mucogingival junction and free gingival groove by 26 gauge needle and the measurement was assessed with a digital caliper) and by ultrasonography (B Scan ultrasonic diagnostic equipment with a frequency of 11 MHz) [15,16].
Surgical Therapy: Patients were subjected to surgical protocol under aseptic conditions after the satisfactory response to Phase I therapy. They were asked to commence rinsing with 10 mL of 0.2% chlorhexidine gluconate twice daily, 24 hours prior to surgery. On the day of the surgery, extraoral surface was swabbed with 5% povidone iodine solution. Patient was then asked to rinse the mouth with 10 mL of 0.2% chlorhexidine digluconate solution for one minute. After achieving adequate local anesthesia at the site, the flap was then designed with different incisions. To the mesial and distal surface of the recession defect, two horizontal bevelled incisions about 3 mm in length were given. These incisions were located at a distance equal to the depth of recession plus 1 mm from the tip of anatomical papillae. At the end of these two horizontal incisions, two bevelled oblique and slightly divergent incisions extending to alveolar mucosa were given. The resulting trapezoidal shaped flap was elevated in the coronal-apical direction. A split thickness flap was elevated between surgical papillae while keeping the blade parallel to the root. A full thickness flap was elevated at the soft tissue lying apical to exposed root. This lead to exposure of around 3-4 mm of bone apical to bone dehiscence. The elevation of flap continued to split thickness till apical to exposure of bone. It was completed once flap could be moved passively in coronal direction. Insertions of all muscles present were removed to facilitate advancement of flap to a level coronal to CEJ of tooth with recession. Connective tissue beds were created by de-epithelialisation of anatomic interdental papilla coronal to horizontal incisions [17]. The root surface was mechanically treated with the use of curettes and was conditioned with EDTA for two minutes to remove smear layer and then thoroughly rinsed with normal saline. Desired width and length of amnion and chorion membranes was placed over defect covering root surface in Group A and Group B sites, respectively. By moving the flap coronally to reach the tip of the de-epithelised anatomical papillae, the vestibular soft tissue was positioned 1 mm coronal to the CEJ to account for soft tissue shrinkage and then was secured with horizontal and vertical sutures using a 5-0 non resorbable suture. Periodontal pack was placed to protect surgical site [Table/Fig-2]. Patient was discharged with postoperative instructions, medications like Amoxicillin 500 mg thrice daily for five days as antibiotic coverage and diclofenac sodium twice daily for five days as anti-inflammatory drug were prescribed. The patient was asked to rinse with chlorohexidine twice daily for two weeks.
Clinical photograph showing surgical procedure of placement of membranes in gingival recession defects: A) Horizontal incision; B) Sulcular incision; C) Vertical incision; D) Reflection of flap; E) Root biomodification; F) Placement of membrane; G) Placement of sutures; H) Application of pack.
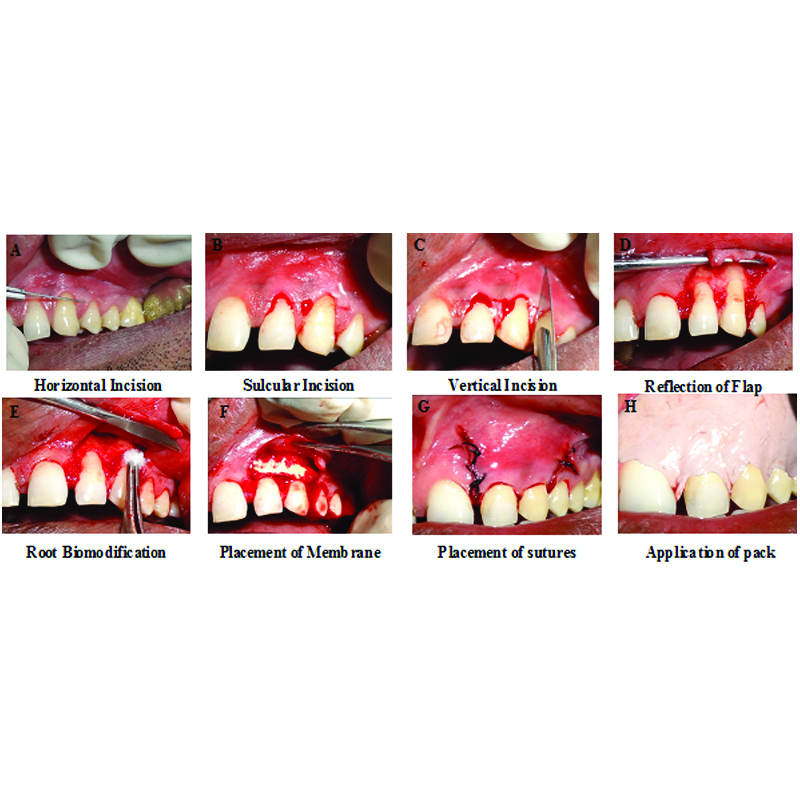
Post-Surgical Follow up: Patients were recalled 24 hours after surgery to evaluate the signs of postoperative complications like pain, discomfort, swelling, hematoma and haemorrhage. After 10 days, the periodontal pack and sutures were removed and the surgical site was thoroughly irrigated with normal saline. Patients were then periodically monitored at three and six months after surgery. On recall visits, oral hygiene was assessed and oral hygiene instructions were reinforced.
Statistical Analysis
All the clinical parameter values thus obtained were then statistically analysed. The mean and standard deviations were calculated for the requisite assessment intervals. For the intragroup comparisons, ‘paired t-test was used and for intergroup comparisons independent t-test was applied. A statistical significance p-value was considerd <0.05.
Results
The mean difference of plaque index and gingival index from baseline to six months for both groups was statistically highly significant [Table/Fig-3].
Mean and mean differences in plaque index and gingival index of Group A and Group B at different intervals and its comparison.
| Assessment interval | Plaque Index | Gingival Index |
|---|
| Mean±SD | Mean difference from baseline | t-value | p-value | Mean±SD | Mean difference from baseline | t-value | p-value |
|---|
| Group A | Baseline | 1.50±0.40 | - | - | - | 1.15±0.45 | - | - | - |
| 3 months | 0.32±0.12 | 1.17±0.37 | 9.94 | <0.001** | 0.10±0.12 | 1.05±0.38 | 8.57 | <0.001** |
| 6 months | 0.42±0.35 | 1.07±0.40 | 8.31 | <0.001** | 0.45±0.32 | 0.70±0.30 | 7.20 | <0.001** |
| Group B | Baseline | 1.57±0.42 | - | - | - | 1.15±0.37 | - | - | - |
| 3 months | 0.27±0.15 | 1.30±0.37 | 10.5 | <0.001** | 0.12±0.13 | 1.02±0.29 | 10.83 | <0.001** |
| 6 months | 0.37±0.35 | 1.20±0.40 | 9.37 | <0.001** | 0.45±0.34 | 0.70±0.42 | 5.25 | <0.001** |
| Group A v/s B | Baseline-3 months | - | 0.13±0.17 | 0.76 | 0.45 | - | 0.02±0.15 | 0.16 | 0.87 |
| Baseline-6 months | - | 0.12±0.18 | 0.68 | 0.50 | - | 0.00±0.16 | 0.00 | 1.00 |
For the intragroup comparisons paired t-test was used and for intergroup comparisons independent t-test was applied
The difference between the probing pocket depth measured at baseline to six months post operatively for both the groups was not statistically significant. Amnion group (Group A) showed statistically significant mean gain in relative clinical attachment, highly significant mean reduction in position of gingival margin and no significant mean increase in the width of keratinised gingiva in six months. Chorion group (Group B) showed statistically highly significant mean gain in the relative clinical attachment level and significant mean reduction in the position of gingival margin at six months [Table/Fig-4,5]. Both the groups showed statistically highly significant mean increase in the gingival thickness measured by both manual and ultrasonographic methods, after six months postoperatively [Table/Fig-6,7]. The Pearson’s correlation coefficient between gingival thickness measured by manual and ultrasonographic method was 0.94, which was statistically highly significant (p-value <0.001) [Table/Fig-8].
Clinical photograph showing assessment of different parameters preoperatively and postoperatively. (A1, B1) shows preoperative probing pocket depth. (A2, B2) shows postoperative probing pocket depth at six months. (A3, B3) shows preoperative relative clinical attachment level. (A4, B4) shows postoperative relative clinical attachment level at six months. (A5, B5) shows preoperative position of gingival margin. (A6, B6) shows postoperative position of gingival margin at six months.
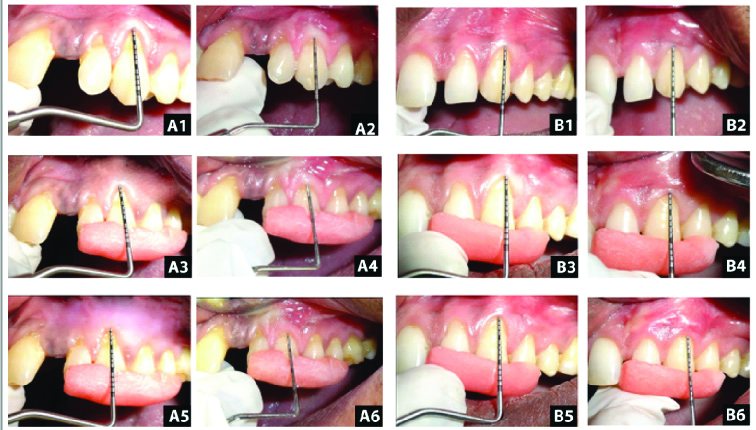
Mean & Mean differences in Probing pocket depth, Relative clinical attachment level and Position of gingival margin Group A & Group B at different intervals and its comparison.
| | Probing Pocket Depth | Relative Clinical Attachment Level | Position Of Gingival Margin |
|---|
| Assessment interval | Mean±SD | Mean difference from baseline | t-value | p-value | Mean±SD | Mean difference from baseline | t-value | p-value | Mean±SD | Mean difference from baseline | t-value | p-value |
|---|
| Group A | Baseline | 1.40±0.69 | - | - | - | 9.60±2.22 | - | - | | 8.00±1.56 | - | - | - |
| 3 months | 1.10±0.31 | 0.30±0.82 | 1.15 | 0.27 | 8.50±2.01 | 1.10±1.19 | 2.90 | 0.01* | 7.00±1.69 | 1.00±0.94 | 3.35 | 0.001** |
| 6 months | 1.10±0.31 | 0.30±0.82 | 1.15 | 0.27 | 8.50±2.01 | 1.10±1.19 | 2.90 | 0.01* | 7.00±1.69 | 1.00±0.94 | 3.35 | 0.001** |
| Group B | Baseline | 1.30±0.48 | - | - | - | 9.50±1.50 | - | - | - | 7.90±1.52 | - | - | - |
| 3 months | 1.10±0.31 | 0.20±0.63 | 1.00 | 0.34 | 8.40±1.34 | 1.10±0.73 | 4.71 | 0.001** | 7.10±1.37 | 0.80±1.03 | 2.44 | 0.03* |
| 6 months | 1.10±0.31 | 0.20±0.63 | 1.00 | 0.34 | 8.40±1.34 | 1.10±0.73 | 4.71 | 0.001** | 7.10±1.37 | 0.80±1.03 | 2.44 | 0.03* |
| Group A v/s B | Baseline-3 months | - | 0.10±0.32 | 0.30 | 0.76 | - | 0.00±0.44 | 0.00 | 1.00 | - | 0.20±0.44 | 0.45 | 0.65 |
| Baseline-6 months | - | 0.10±0.32 | 0.30 | 0.76 | - | 0.00±0.44 | 0.00 | 1.00 | - | 0.20±0.44 | 0.45 | 0.65 |
For the intragroup comparisons, ‘paired t-test’ was used and for intergroup comparisons ‘independent t-test’ was applied
Photograph showing measurement of gingival thickness (A) manually and (B) by ultrasonographic method
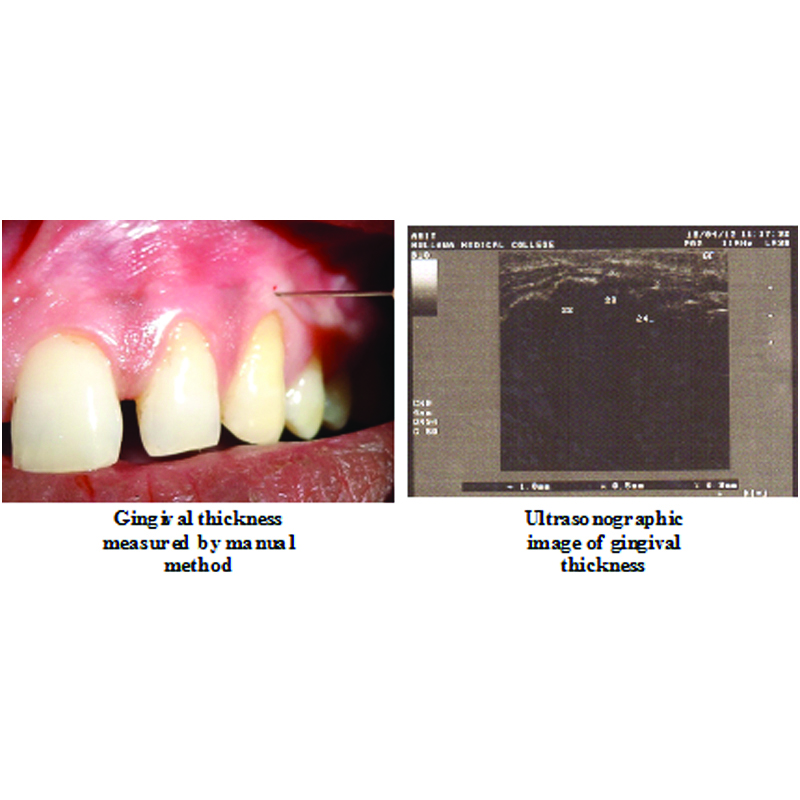
Mean and mean differences in Gingival thickness measured by manual and ultrasonography method of Group A and Group B at different intervals and its com-parison.
| Gingival thickness measured by manual method | Gingival thickness measured by ultrasonography |
|---|
| Group | Assessment interval | Mean±SD | Mean difference from baseline | t-value | p-value | Mean±SD | Mean difference from baseline | t-value | p-value |
|---|
| Group A | Baseline | 0.67±0.19 | - | - | - | 0.55±0.20 | - | - | - |
| six month | 1.27±0.17 | 0.59±0.22 | 8.31 | <0.001** | 1.02±0.17 | 0.47±0.26 | 5.56 | <0.001** |
| Group B | Baseline | 0.59±0.13 | - | | - | 0.46±0.14 | - | - | - |
| six month | 1.22±0.21 | 0.63±0.26 | 7.62 | <0.001** | 1.00±0.24 | 0.54±0.28 | 5.93 | <0.001** |
| Group A v/s B | Baseline-six month | - | 0.04±0.11 | 0.36 | 0.72 | - | 0.07±0.12 | 0.56 | 0.58 |
For the intragroup comparisons, ‘paired t-test’ was used and for intergroup comparisons ‘independent t-test’ was applied
Correlation coefficient between gingival thickness measured by manual and ultrasonography method.
| Method Used | Test Applied | Gingival Thickness Measured By Manual Method | Gingival Thickness Ultrasonography |
|---|
| Gingival Thickness Measured By Manual Method | Pearson’s correlation | 1 | 0.944** |
| p-value | | <0.001 |
| Gingival Thickness Ultrasonography | Pearson’s correlation | 0.944** | 1 |
| p-value | <0.001 | |
**Correlation is significant at the 0.01 level, Pearson coefficient correlation
Discussion
Conventional mucogingival procedures such as the laterally positioned flap, free gingival graft, connective tissue graft, and coronally positioned flap have been shown to be relatively successful in achieving root coverage. However, in case of free gingival graft, the results are not always aesthetically satisfactory. The drawbacks inherent with autografts like colour discrepancy between the graft and the surrounding tissue in case of free gingival grafts and bulky tissue contours in case of connective tissue grafts have lead to a search for alternative approaches.
According to Allen EP, the CAF is the most aesthetically effective mucogingival procedure for correcting localised gingival recessions [18]. In addition, there is no need for a second surgical site, like with a free gingival or connective tissue graft [19]. However, this procedure does not increase the width of the keratinised gingiva and provides little or no periodontal regeneration in gingival recession defects [20]. A variety of materials have been used with CAF to give different results. In this study, human placental amnion and chorion membranes were used because of their various properties which make them quite unique.
Collagen layers of amnion and chorion are rich in Type I, IV, V, VI collagen, proteoglycans, laminin and fibronectin [9]. These membranes possess antibacterial and antimicrobial properties [10], reduce inflammation at the wound site by the presence of natural inhibitors of matrix metalloproteinases-1,2,3,4, interleukin-10 and interleukin-1 receptor antagonists [11], cause suppression of Interleukin 1α and 1β [21]. Hyaluronic acid present in the amnion membrane causes entrapment and adhesion of inflammatory cells including lymphocytes to the amniotic membrane stroma [22, 23]. The membranes also express antimicrobial peptides like β-defense and produce elastase inhibitors like secretory leukocyte proteinase inhibitor and elafin [24-26]. The placental membranes have been used in the past as a surgical wound dressing, treatment of leg ulcers, skin loss in Stevens-Johnsons diseases, reconstruction of the pelvic floor, vaginal epithelialisation, replacement of normal mucosa in Rendu Osler-Weber diseases, ear surgery and for the treatment of various corneal defects [27,28].
There is paucity in literature regarding the use of these membranes in the field of periodontics. Favourable results have been found with these membranes by Gurinsky B in root coverage procedure, Wallace S for guided bone regeneration, Arai N et al., as intraoral wound dressing material and Rosen PS for correcting both hard and soft tissue deformities around maxillary canine [29-32]. Few studies have been conducted recently to evaluate the application of these membranes for treatment of gingival recession [Table/Fig-9] [33-41].
List of recent studies for evaluation of placental membranes for gingival recession defects.
| Authors | Year of Study | Study Title |
|---|
| Singh H and Singh H [33] | 2013 | Bioactive amnion as a Guided Tissue Regeneration (GTR) membrane for treatment of isolated gingival recession: a case report. |
| Holtzclaw DJ and Toscano NJ [34] | 2013 | Amnion-chorion allograft barrier used for guided tissue regeneration treatment of periodontal intrabony defects: a retrospective observational report. |
| Shetty SS, et al., [35] | 2014 | Bilateral multiple recession coverage with platelet-rich fibrin in comparison with amniotic membrane. |
| Shah R, et al., [36] | 2014 | Amnion membrane for coverage of gingival recession: a novel application. |
| Chakraborthy S, et al., [37] | 2015 | Amnion and chorion allografts in combination with coronally advanced flap in the treatment of gingival recession: a clinical study. |
| Sharma A and Yadav K [38] | 2015 | Amniotic membrane-a novel material for the root coverage: a case series. |
| Pundir AJ, et al., [39] | 2016 | Comparative evaluation of the efficacy of human chorion and amnion with coronally advanced flap for recession coverage: a case series. |
| Agarwal SK, et al., [40] | 2016 | Patient-centered evaluation of microsurgical management of gingival recession using coronally advanced flap with platelet-rich fibrin or amnion membrane: a comparative analysis. |
| Elzanaty M, et al., [41] | 2017 | Clinical evaluation of amnion chorion membrane in comparison to subepithelial connective tissue graft in gingival recession coverage. |
Thus, based on the properties of these placental membranes, this study was conducted to evaluate the clinical efficacy of the membranes in gingival recession defects and their effect on gingival biotype.
In this study, a total of twenty sites from ten patients, with gingival recession defects were selected and divided into Group A (CAF with amnion membrane) and Group B (CAF with chorion membrane) according to the treatment modality received.
All the selected sites were subjected to assessment of the clinical parameters like plaque index, gingival index, probing pocket depth, relative clinical attachment level, position of gingival margin and gingival thickness measured by manual method and by ultrasonography both pre and post operatively at different intervals.
There was statistically no significant difference between the probing pocket depth measured at baseline to six months postoperatively for both the groups (p-value of 0.27 and 0.34 for Group A and Group B respectively). These findings compare well with studies of Prato PG et al., indicating that increase in buccal probing depth is not a common side effect following root coverage procedures [42].
Both the groups showed statistically significant gain in the clinical attachment level with Group A mean gain of 1.10±1.19 mm and Group B mean gain of 1.10±0.73 mm in the relative clinical attachment level at six months postoperatively from the baseline (p-value <0.001). Chakraborthy S et al., also observed gain in attachment level of 2.17±1.53 mm for amnion and 1.58±1.22 mm for chorion site in their study [37]. Holtzclaw DJ and Toscano NJ observed an improvement of 4.61-1.29 mm in clinical attachment level when they used amniotic membrane as a barrier membrane in the treatment of periodontal intrabony defects [34].
Wallace SC also reported gain in clinical attachment level of 2.4 mm with placental membranes [30]. On contrary, Sharma A and Yadav K observed a decrease in clinical attachment level from 6.4±0.54 mm preoperatively to 3.5±0.9 mm postoperatively at six months but a significant improvement in keratinised gingiva tissue width from 3.2±0.28 mm preoperatively to 5.9±0.74 mm postoperatively at six months [38].
In this study, Group A sites showed statistically highly significant mean reduction in the position of gingival margin by 1.00±0.94 (p-value <0.001) and Group B showed statistically significant mean reduction in the position of gingival margin by 0.80±1.03 (p-value <0.03) at three and six months postoperatively from the baseline which was in accordance with studies done by Zahedi S et al., who used diphenylphosphorylazide cross-linked collagen membrane and showed a mean reduction in recession depth by 2.9 mm [43]. Wang HL et al., Kimble KM et al., and Trabulsi M et al., who used collagen membrane also showed a mean reduction in gingival recession depth by 2.5 mm, 2.1 mm and 2.38 mm respectively at six months [44-46]. On comparison between the mean difference of Group A and Group B at three months and six months from the baseline, the results were statistically non significant (p-value =0.65).
In this study, the gingival thickness was assessed by both manual and ultrasonographic methods for both the groups. The results obtained at six months were statistically highly significant. A statistically highly significant mean increase in the gingival thickness at six months postoperatively from the baseline with mean increase of 0.59±0.22 mm (p-value <0.001) and 0.63±0.26 mm (p-value <0.001) was observed for Group A and Group B, respectively. On comparison between the mean difference of two groups at six months from the baseline, the results were statistically non significant (p-value =0.72). In this study, ultrasound was used to measure the gingival thickness as it measures the gingival thickness rapidly and atraumatically. Authors have measured the reliability and validity of ultrasonic measurements by using both A scan and B scan ultrasonic devices. Eger T et al., measured the thickness of attached gingiva using a commercially available A-mode intraoral ultrasonic device and reported that the validity and reliability of measuring gingival thickness with the ultrasonic device was excellent [47]. Uchida H, et al., obtained the thickness of masticatory mucosa by application of the B-mode ultrasonic method and concluded that this technique may be useful for in vivo determination of the degree of soft tissue displacement under dentures by occlusal forces [16]. Savitha B and Vandana KL used ultrasound A-scan and concluded that it is reliable in measuring gingival thickness mid-buccally [15]. In this study, B scan ultrasonic device was used to measure the gingival thickness and on observation Group A and Group B showed statistically highly significant mean increase in the gingival thickness at six months postoperatively from the baseline with mean increase of 0.47±0.26 mm (p-value <0.001) and 0.54±0.28 mm (p-value <0.001) respectively. On comparison between the mean difference of Group A and Group B at six months from the baseline, the results were statistically non significant (p-value =0.56). The correlation between the manual method and the ultrasonographic method showed highly significant relation (p-value <0.001) indicating that the variation between both the methods from baseline to six months postoperatively was same.
Thick gingival tissue is probably the image most associated with periodontal health by Kao RT and Pasquinelli K [48]. For the treatment of gingival recession, gingival biotype is an essential modifying factor. Baldi C et al., concluded that in root coverage procedures, an initial flap thickness of 0.8 mm to 1.2 mm was the most significant factor associated with a complete root coverage procedure [49]. A high association between flap thickness and complete root coverage has also been reported in a meta-analysis by Hwang D and Wang HL [8]. Complete root coverage with amniotic membranes in gingival recession defects was also observed by Shetty SS et al., and Shah R et al., [35,36]. Agarwal SK et al., also observed an enhancement in root coverage when amniotic membrane was used in conjunction with CAF as compared to CAF alone [40]. Singh H and Singh H showed significant root coverage with uneventful healing in a case treated with bioactive amniotic membrane for isolated gingival recession [33]. Elzanaty M et al., observed root coverage of 80.83% with the amnion chorion allograft along with CAF at six months in gingival recession defect [41]. Chakraborthy S et al., observed 34% of root coverage for chorion site and 22% for amnion site and Sharma A and Yadav K observed a mean root coverage of 70.2±6.8% [37,38]. In a study by Pundir AJ et al., 9 of 12 treated recession defects showed 100% root coverage. The gingival biotype also showed a thick biotype in 10 sites that had an initial thin biotype [39].
Limitation
The present study was carried out for a period of six months which is a short period to fully evaluate the effect of root coverage procedures. The comparison between the coronally advanced flap procedure alone and with the use of membranes should be established in the future studies. Histological analysis which is essential to confirm periodontal regeneration occurring in these membrane based root coverage procedures was not performed in this study.
Conclusion
Based on overall clinical observations, both amnion (Group A) and chorion (Group B) showed good results in the treatment of gingival recession defects as root coverage procedures, with a statistically significant mean gain in the relative clinical attachment level, position of gingival margin as well as gingival thickness. On comparison, there was no statistically significant difference observed between both the groups. Mean increase in the gingival thickness with chorion group (Group B) was slightly more but the difference was not statistically significant. The observations made by the various clinical parameters suggest that both the membranes can be used in the treatment of gingival recession defects and to augment the gingival biotype. More researches with an extensive study period and a larger sample size are needed to be carried out to assess the long term stability of the results with these membranes. Histological analysis is also essential to conclude whether periodontal regeneration has occurred with these membrane based root coverage procedures.
For the intragroup comparisons paired t-test was used and for intergroup comparisons independent t-test was applied
For the intragroup comparisons, ‘paired t-test’ was used and for intergroup comparisons ‘independent t-test’ was applied
For the intragroup comparisons, ‘paired t-test’ was used and for intergroup comparisons ‘independent t-test’ was applied
**Correlation is significant at the 0.01 level, Pearson coefficient correlation