Prolonged Remission and Good Quality of Life with Maintenance Chemotherapy in Recurrent Ewing’s Sarcoma
Priyanka Sreelatha1, Wesley Mannirathil Jose2
1 Student, Department of Pharmacy Practice, Amrita Institute of Medical Sciences, Amrita Vishwa Vidyapeetham, Amrita University, Kochi, Kerala, India.
2 Associate Professor, Department of Oncology, Amrita Institute of Medical Sciences, Amrita Vishwa Vidyapeetham, Amrita University, Kochi, Kerala, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Wesley Mannirathil Jose, Associate Professor, Department of Medical Oncology and Haematology, Cancer Institute, Amrita Institute of Medical Sciences, Amrita Vishwa Vidyapeetham, Amrita University, Kochi-682041, Kerala, India.
E-mail: wesleymjose@aims.amrita.edu
Ewing’s sarcoma is an aggressive tumour of bone commonly affecting individuals in 10-20 years of age group. Older age and metastatic disease has an overall poor outcome. We are reporting a case of a 37-year-old lady with Ewing’s sarcoma who was initially diagnosed with localised disease involving C5-7 vertebral bodies. She received conventional treatment. She relapsed three years later with metastatic disease and was treated with salvage treatment followed by high dose chemotherapy and autologous stem cell transplant. She had a second relapse with lung metastasis after 28 months. She was treated with gemcitabine and docetaxel and she achieved complete remission again. She is on maintenance chemotherapy with cyclophosphamide and etoposide and continues to be in remission on maintenance treatment.
Lung metastasis, Neuroectodermal tumour, Recurrent sarcoma
Case Report
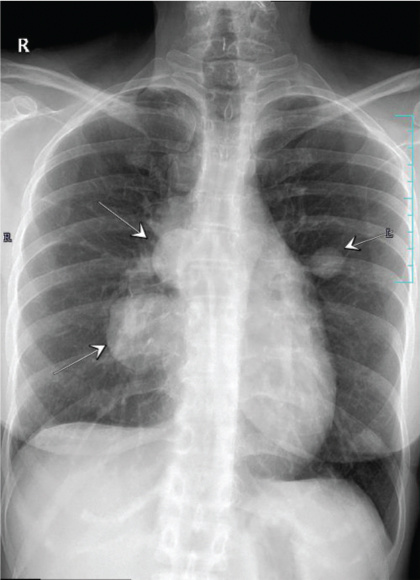
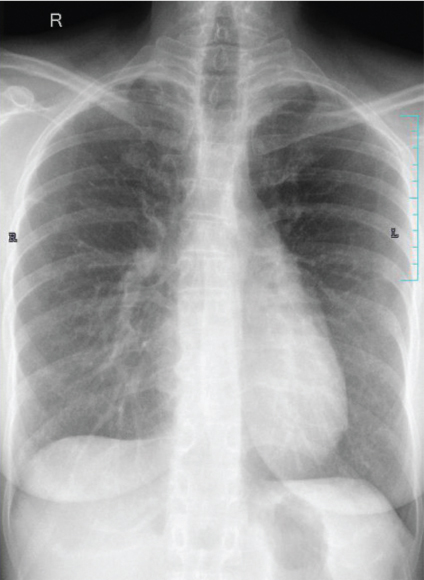
A 37-year-old Indian woman, presented with severe neck pain radiating to the arms, in 2007 at the age of 27 years. Magnetic Resonance Imaging (MRI) cervical spine revealed an enhancing epidural soft tissue lesion in the cervical spinal canal extending from C4-T1 level with abnormal signals in C5, C6, C7 vertebral bodies. Biopsy was suggestive of Primitive Neuroectodermal Tumour (PNET)/extraskeletal Ewing’s sarcoma. Her initial metastatic work up including bone marrow and skeletal scintigraphy was negative. She had an upfront debulking surgery following which her neck pain subsided. Thereafter, she was administered adjuvant chemotherapy with EURO Ewing protocol followed by consolidative radiation of 5000 centigray (cGy) in 25 fractions to the site of primary disease which she completed in late 2008. Three years later she presented in December 2011 with progressive cough and shortness of breath of one month duration. Computed Tomography (CT) scan of chest revealed right sided moderate pleural effusion and multiple pleural nodules. Her pleural fluid cytology was positive for malignant cells. She was administered three cycles of salvage chemotherapy with ifosfamide, carboplatin and etoposide. She attained a very good partial response and therefore was taken up for high dose chemotherapy and Autologous Stem Cell Transplantation (ASCT) in early 2012. She was given conditioning with busulfan-melphalan. She achieved complete remission and remained on close follow up surveillance for next 28 months. She presented in mid 2014 with persistent cough of two weeks duration. Chest X-ray and Positron Emission Tomography (PET-CT) showed bilateral canon ball lesions in both lungs [Table/Fig-1]. In view of her young age and good performance status, she was initiated on salvage chemotherapy with gemcitabine and docetaxel. She completed six cycles of the same and achieved radiological complete remission. Due to multiple recurrences, in an attempt to prolong her disease free interval, she was initiated on maintenance chemotherapy with cyclophosphamide 50 mg Once a Day (OD) and etoposide 50 mg OD (14/28 days). At the time of reporting she has completed 32 months of maintenance chemotherapy and continues to be in complete remission as evidenced by surveillance chest X-ray and PET-CT [Table/Fig-2]. She leads a fully active life.
Bilateral canon ball lesions in lungs clearly visible in chest X-ray (marked by white arrows).
Chest X-ray showing complete remission.
Discussion
Ewing’s sarcoma is an aggressive tumour of bone with incidence of 2.93 cases/1,000,000 population and relatively less frequent in African and Asian population [1,2]. The median age of patients with Ewing’s sarcoma is 15 years. Patients above 15 years of age generally have a poorer outcome. This patient was 27-year-old at the time of diagnosis.
The Intergroup Ewing’s Sarcoma Study (IESS) which was initiated in 1973 provided unequivocal evidence in favour of systemic chemotherapy in treatment of Ewing’s sarcoma [3]. The current standard of care for non metastatic Ewing’s includes chemotherapy with vincristine, doxorubicin, ifosfamide and etoposide for nearly 42-48 weeks with local control in form of surgery and radiation therapy administered at 12-15 week. This patient had received the standard treatment. Even after a successful primary treatment, relapse is common and may occur years later.
Five year relapse free survival for patients without metastasis at diagnosis is 55% and the five year overall survival rate for patients with metastatic or recurrent Ewing’s sarcoma remains less than 20% [4]. Patients who relapse in first two years fare worse with five year survival of 7% [5]. This patient had her first relapse after three years. She has survived for nearly six years after diagnosis of metastatic disease. Patients with pulmonary recurrence or metastasis have a relatively better outcome compared to those with bone or other sites of metastasis [6]. Since, this patient had lung only metastasis and had her first relapse after three years, these could be considered favourable factors leading to a better outcome.
In the absence of prospective randomised studies, there is no defined standard of care for relapsed disease. In phase 1 and 2 studies, irinotecan with temozolomide and gemcitabine with docetaxel have been found to be effective [7,8]. Beyond these conventional chemotherapeutic agents, the treatment options for a patient with relapse and recurrent disease is scarce. This patient received gemcitabine and docetaxel as the salvage regimen.
In order to improve outcomes, strategies such as maintenance therapy and long term treatment with targeted therapy, are being investigated in patients with advanced sarcomas. By convention each subsequent disease free interval would be shorter than the previous one. In this case, the last disease free interval of 32 months is longer than the one achieved even with intense treatment regimen and autologous stem cell transplant. Therefore, we believe that maintenance treatment prescribed to this patient is the sole cause of her ongoing response and excellent quality of life. Mammalian Target of Rapamycin (mTOR) inhibitors for maintenance therapy is one such promising drug. Ridaforolimus (AP23573, MK-8669), an mTOR inhibitor, is currently being evaluated in the ongoing Sarcoma mUlti-Center Clinical Evaluation of the Efficacy of riDaforolimus (SUCCEED) trial [9].
There is great need for newer therapies in management of Ewing’s sarcoma. A large number of studies are ongoing in this field. A few prominent ones are mentioned here with their respective clinical trial numbers in parenthesis. The details of these trials can be accessed in the National Cancer Institute website [10].
Ewing’s sarcoma is defined by the presence of unique EWS-FLI fusion protein which is critical to the maintenance of its oncogenicity. Targeting this protein has been central to the research efforts over past two decades. YK-4-279 is a small peptide that acts by blocking the interaction of EWS/FLI with RNA helicase A resulting in cell cytotoxicity and reduced EWS/FLI-driven transcriptional activation [11]. This small peptide has shown preclinical activity in Ewing’s sarcoma and a phase 1 dose escalation study of intravenous TK216 (clinical derivative of YK-4-279) in patients with relapsed or refractory Ewing’s sarcoma (Clinicaltrials.gov ID NCT02657005) is currently recruiting patients.
The attempts at reversing EWS/FLI-driven gene signatures and thereby blocking the malignant propensity of Ewing’s sarcoma with the help of camptothecins have not achieved much clinical success as single agents [12].
Poly ADP Ribose Polymerase (PARP) is a superfamily of multifunctional enzymes and PARP-1 is found to be highly expressed in Ewing’s tumour. Talazoparib (NCT02116777), niraparib (NCT02044120), olaparib (NCT01858168; NCT02398058) in combination with other cytotoxic agents are presently under study in phase 1 and 2 trials for this disease [10].
Small molecule multi receptor tyrosine kinase inhibitors like pazopanib (NCT01956669), regorafenib (NCT02048371), cabozantinib (NCT02243605) is being studied for its efficacy in treatment of relapse recurrent Ewing’s sarcoma. CDK4/6 inhibitor abemaciclib (NCT02644460) and PD-1 inhibitor nivolumab (NCT02304458), too are in early phase 1 and 2 trials [10].
A large number of newer molecular agents (T-cell and NK cell based immunotherapy, cancer vaccines, monoclonal antibodies etc.) is being studied and many promising molecules are under development [13].
Recently, Food and Drug Administration (FDA) granted an orphan drug designation to ganitumab to treat Ewing’s sarcoma. Ganitumab inhibits malignant cell proliferation through disruption of the PI3k/AKT and Mitogen-Activated Protein Kinases (MAPK) pathways. A phase 3 clinical trial (NCT02306161) is ongoing [14].
Until such time that a treatment is found to be definitely efficacious and affordable, the benefit of survival may not reach the common patient. Moreover, in resource constrained settings, like India and other developing nations, utilisation of the newer agents has significant limitations.
In the case presented here, the patient had multiple recurrences and poor quality of life and added financial burden which prompted the need for considering maintenance chemotherapy after achieving a complete remission. There are no standard drugs recommended for maintenance therapy in recurrent Ewing’s sarcoma. We used the combination of cyclophosphamide and etoposide since the patient had shown clinical response to their use in her initial treatment phase. Cyclophosphamide and etoposide also gives convenience of oral administration with minimal toxicities and good tolerance [15]. This provides for reduced hospital visits and better quality of life.
Conclusion
Maintenance chemotherapy may have a role in the management of metastatic or recurrent Ewing’s sarcoma and needs to be studied in a randomised trial. Until the newer agents are approved and made available, cyclophosphamide and etoposide or any other combination of drugs to which patient has previously responded are good options.
[1]. Esiashvili N, Goodman M, Marcus RB, Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: surveillance epidemiology and end results dataJ Pediatr Hematol Oncol 2008 30(6):425-30.10.1097/MPH.0b013e31816e22f318525458 [Google Scholar] [CrossRef] [PubMed]
[2]. Worch J, Matthay KK, Neuhaus J, Goldsby R, DuBois SG, Ethnic and racial differences in patients with Ewing SarcomaCancer 2010 116(4):983-88.10.1002/cncr.2486520052725 [Google Scholar] [CrossRef] [PubMed]
[3]. Nesbit ME, Perez CA, Teft M, Burgert EO, Vietti TJ, Kissane J, Multimodal therapy for the management of primary, nonmetastatic Ewing’s sarcoma of bone: an intergroup studyNational Cancer Institute Monograph 1981 56:255-62. [Google Scholar]
[4]. Cotterill SJ, Ahrens S, Paulussen M, Jurgens HF, Voute PA, Gadner H, Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European intergroup cooperative Ewing’s sarcoma study groupJ Clin Oncol 2000 18(17):3108-14.10.1200/JCO.2000.18.17.310810963639 [Google Scholar] [CrossRef] [PubMed]
[5]. Leavey PJ, Mascarenhas L, Marina N, Chen Z, Krailo M, Miser J, Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi-modality therapy: a report from the children’s oncology groupPediatr Blood Cancer 2008 51(3):334-38.10.1002/pbc.2161818506764 [Google Scholar] [CrossRef] [PubMed]
[6]. Rodriguez Galindo C, Billups CA, Kun LE, Rao BN, Pratt CB, Merchant TE, Survival after recurrence of Ewing tumors: the St Jude Children’s Research Hospital experienceCancer 2002 94(2):561-69.10.1002/cncr.1019211900241 [Google Scholar] [CrossRef] [PubMed]
[7]. Wagner LM, McAllister N, Goldsby RE, Rausen AR, McNall-Knapp RY, McCarville MB, Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcomaPediatr Blood Cancer 2007 48(2):132-39.10.1002/pbc.2069716317751 [Google Scholar] [CrossRef] [PubMed]
[8]. Fox E, Patel S, Wathen JK, Schuetze S, Chawla S, Harmon D, Phase II study of sequential gemcitabine followed by docetaxel for recurrent Ewing sarcoma, osteosarcoma, or unresectable or locally recurrent chondrosarcoma: results of sarcoma alliance for research through collaboration study 003Oncologist 2012 17(3):32110.1634/theoncologist.2010-026522363068 [Google Scholar] [CrossRef] [PubMed]
[9]. Demetri GD, Chawla SP, Ray-Coquard I, Le Cesne A, Staddon AP, Milhem MM, Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapyJ Clin Oncol 2013 31(19):2485-92.10.1200/JCO.2012.45.576623715582 [Google Scholar] [CrossRef] [PubMed]
[10]. National Cancer Institute. (2017). Clinical trials search results. [online] Available at: https://www.cancer.gov/about-cancer/treatment/clinical-trials/search/r?q=Ewings+sarcoma+&t=&a=&z=&rl=1 [Accessed 16 Nov. 2017] [Google Scholar]
[11]. Barber-Rotenberg JS, Selvanathan SP, Kong Y, Erkizan HV, Snyder TM, Hong SP, Single enantiomer of YK-4-279 demonstrates specificity in targeting the oncogene EWS-FLI1Oncotarget 2012 3(2):172-82.10.18632/oncotarget.45422383402 [Google Scholar] [CrossRef] [PubMed]
[12]. Baruchel S, Pappo A, Krailo M, Baker KS, Wu B, Villaluna D, A phase 2 trial of trabectedin in children with recurrent rhabdomyosarcoma, Ewing sarcoma and non-rhabdomyosarcoma soft tissue sarcomas: a report from the children’s oncology groupEur J Cancer 2012 48(4):579-85.10.1016/j.ejca.2011.09.02722088484 [Google Scholar] [CrossRef] [PubMed]
[13]. Yu H, Ge Y, Guo L, Huang L, Potential approaches to the treatment of Ewing’s sarcomaOncotarget 2017 8(3):5523-39.10.18632/oncotarget.1256627740934 [Google Scholar] [CrossRef] [PubMed]
[14]. Fdanews.com. (2017). FDA grants orphan drug designation for nantcell’s ganitumab in Ewing sarcoma. [online] Available at: https://www.fdanews.com/articles/181381-fda-grants-orphan-drug-designation-for-nantcells-ganitumab-in-ewing-sarcoma [Accessed 16 Nov. 2017] [Google Scholar]
[15]. Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, Addition of ifosfamide and etoposide to standard chemotherapy for ewing’s sarcoma and primitive neuroectodermal tumor of boneN Engl J Med 2003 348(8):694-701.10.1056/NEJMoa02089012594313 [Google Scholar] [CrossRef] [PubMed]