The role of microorganisms in the aetiology of pulp and periapical diseases has been emphasised in various studies and for a successful endodontic outcome their control and elimination are important. Chemomechanical preparation removes majority of infecting bacteria, together with necrotic pulp debris [1]. However, due to anatomical complexity and the limitation in accessing the canal by instruments and irrigants complete disinfection is not always achieved. Persistent endodontic infection might be attributed to the retention of microorganisms in dentinal tubules [2].
Intracanal medicaments help in reducing the bacteria remaining even after the chemomechanical preparation and can provide an favourable environment for periapical healing [3]. E. faecalis, a Gram positive and facultative anaerobe, is frequently found in cases of re-infection and its prevalence range from 24-77% [4]. Stressful conditions like starvation do not affect the persistence of these bacteria in canal space [3]. Traditionally Calcium hydroxide has been used as an intracanal medicament because of its anti-bactericidal properties due to its high pH which has a destructive effect on bacterial cell membranes and protein structure [5]. However, studies have shown its inability to completely eliminate the bacteria from the tubules [6]. Due to the broad spectrum antimicrobial nature, Chlorhexidine, a synthetic cationic bisguanide shows a promising potential use as a root canal irrigant and medicament [7,8]. However, it lacks the tissue dissolving property, which is its major limitation [7].
Due to antimicrobial resistance and side effects that pathogenic micro organisms build against the common antibiotics, recently the extracts from biologically active compounds isolated from plants have received huge attention [11,12].
In the present study, the plant extraction was done by soxhlet technique and to the knowledge of the authors no previous studies have been done using this method. Hence, the objective of this in vitro study was to evaluate and compare the antibacterial efficacy of two medicinal plants neem extract and aloevera with 2% chlorhexidine and Calcium hydroxide against E. faecalis, at intervals of 1, 2 and 5 days.
Materials and Methods
This in vitro study was conducted at the Department of Conservative Dentistry and Endodontics, JSS Dental College and Hospital (JSS Academy of Higher Education and Research), Mysore in association with Department of Microbiology and Department of Pharmacology, JSS Medical College and hospital (JSS Academy of Higher Education and Research), Mysore and was approved by the Institutional Ethical Committee for research on human subjects or specimens.
Fresh neem leaves were collected and shade dried. The leaves were then sieved to obtain coarse powder and a uniform texture. The sieved powder was subjected to hot Soxhlet extraction for 24 hour cycle at 60-65°C. The solvent was completely removed under reduced pressure by Lyotrap dryer (LTE Scientific Ltd, London, UK). The extract was then stored at 4°C in a tightly closed container to preserve it from any contamination and decomposition. The extract was then redissolved in ethanol (0.065 gm/L) to the desired concentration and used [13].
For aloevera, mature healthy and fresh leaves were collected and the inner colourless, mucilaginous pulp was homogenised and centrifuged at 6400 g at 4°C for 15 minute to remove the fibers. The resultant supernatant was lyophilised and sample was extracted with 95% ethanol. The obtained filtrate was collected and evaporated to dryness under reduced pressure of 250 mmHg in a rotary evaporator [14]. The extract was then redissolved in ethanol (0.044 g/μL) to the desired concentration and used.
A 2% Chlorhexidine (RC-Chlor, Azure Lab, Kochi, India) and calcium hydroxide (RC Cal, Prime dental, Mumbai, India) were used in the canals as they were supplied by manufacturer.
Preparation of Dentin Specimens
The model proposed by Haapasalo M and Orstavik D was modified and used in the study [15]. A total of 90 single rooted human mandibular premolar teeth freshly extracted for orthodontic reasons were selected. Teeth were disinfected with 5.25% Sodium Hypochlorite (NaOCl) (Nice Chemicals, Kochi, India). Calculus and tissue remnants were removed using ultrasonic scaler (Varios 750, NSK Nakanishi Inc., Tochigi, Japan). Only teeth with single root and canal were selected for the study. The type I canal configuration was confirmed by using digital radiograph (Oralix AC Gendex, Dentsply, Milano, Italy) in mesio-distal and bucco-lingual planes. Teeth with curvature, pre-existing carious lesions, cracks, fracture, endodontic treatment, resorption, open apices or radiographically invisible canals or multiple canals were excluded from the study. A rotary diamond disc (NTI® Diamond Discs, Axis-Sybronendo, Kerr Corporation CA, USA) was used to decoronate the teeth below the CEJ and the apical part of the root to obtain 6 mm of the middle one third of root. Gates Glidden drill no. 2 (Mani Inc, Tochigi, Japan) in a slow speed handpiece was used to standardise the internal diameter of the root canals [Table/Fig-1]. The specimens were placed in an ultrasonic bath of 17% EDTA (Canalarge, Ammdent, Chandigarh, India) for five minutes followed by 3% NaOCl (Nice chemicals Pvt. Ltd., India) for five minutes to remove organic and inorganic debris. The traces of chemicals used were removed by immersing the dentin specimens in an ultrasonic bath containing distilled water for five minutes. All the specimens were sterilised in an autoclave for two cycles. The first cycle was at 121°C and the second was with the specimens immersed in 1 mL of Brain Heart Infusion (BHI) broth (Himedia Laboratories, Mumbai, India) in individual microcentrifuge tubes.
Prepared specimen (6 mm) showing the internal canal diameter.
Contamination of the Specimens
The test organism used for the study was E. faecalis which is a Gram positive facultative anaerobic bacterium that is common in root filled teeth with post treatment infection. E. faecalis (ATCC 29212) was grown in BHI agar for 24 hour. The culture was suspended in 5 mL of BHI broth and incubated for 4 hour at 37°C and its turbidity was adjusted at 0.5 Mcfarland standard. Each dentin block was placed in pre-sterilised microcentrifuge tubes containing 1 mL of the BHI broth. A 50 μL of the inoculums containing E.faecalis was transferred into each of the microcentrifuge tubes. At the end of 24 hours, the dentin specimens were transferred into fresh broth containing E.faecalis. All procedures were carried out under laminar flow (Thermo Fisher Scientific Inc, Waltham, MA USA). Purity of the culture was checked by subculturing 5 μL of the broth from the incubated dentin specimens in BHI broth on BHI agar plates. Contamination of the dentin specimens was carried out for a period of 21 days.
Antimicrobial Assessment
At the end of 21 days, the specimens were irrigated with 5 mL of sterile saline to remove the incubation broth. They were assigned into five groups (n=18). Group 1: 2% Chlorhexidine (RC-Chlor, Azure Lab, Kochi, India), Group 2: Neem, Group 3: Calcium hydroxide (RC Cal, Prime dental, Mumbai, India), Group 4: Aloevera, Group 5: Saline (Nice Chemicals, Kochi, India). The respective medicaments were placed in root canal and paraffin wax was used to seal both the ends; the specimens were then incubated in an anaerobic environment for 37°C. Microbial cells assessment was carried out at the end of 1, 3 and 5 days of incubation, with six specimens at each intervals of time. Harvesting of dentin was carried out at two depths (200 μm and 400 μm) by preparing the root canal circumferentially using sterile Gates Glidden drills no.3 and no.4 respectively (Mani Inc, Tochigi, Japan) in slow speed handpiece (Mani Inc, Tochigi, Japan) [Table/Fig-2]. The fine dentin shavings were collected in a test tube containing 1 mL of sterile BHI broth and incubated in an anaerobic environment at 37°C for 24 hour. After 24 hour the contents were serially diluted 100 μL of broth in 100 μL of sterile saline five times. About 50 μL of the dilution was then plated on BHI agar plates and incubated for 24 hour at 37°C. Colonies were counted and readings were tabulated.
Inoculation of dentin shavings into BHI broth.

Determination of the Minimum Inhibitory Concentration (MIC) Using Microbroth Dilution Test
To determine the MIC, the dilution test was performed using the standard procedures as explained by Jorgensen JH et al., [16]. A total of 100 μL of BHI broth was added in each well of a μL plate. The 100 μL aliquot of stock solution of crude ethanolic extract i.e., Neem extract (0.065 gm/μL) and aloevera extract (0.044 gm/μL) was added, and subsequently two fold serially diluted. The inoculum suspension (100 μL) of each bacterial strain was then added in each well containing ethanolic extracts and BHI broth. The procedure for negative and positive controls was also performed using 10% ethanol and saline respectively. The plates were incubated at 37°C for 24 hours and the turbidity was measured at 620 nm using the microplate reader (IEMS Reader MF, Lab systems) [Table/Fig-3]. The lowest concentration that inhibited visible growth of the tested organism was recorded as the MIC.
Microtiter plates used for MIC determination, of neem, aloevera, chlorhexidine, calcium hydroxide and saline.

MIC obtained was
Neem extract-0.129 mg/μL
Aleovera extract-2.76 mg/μL
Statistical Analysis
The data were statistically analysed with one-way Analysis of Variance (ANOVA), followed by Scheffe’s multiple comparisons means to check the differences in CFU count between groups (p<0.05). The analysis was performed with Statistical Package for the Social Sciences (SPSS version 16.0, SPSS Inc, Chicago, IL, USA).
Results
The present study showed that all medicaments exerted antibacterial activity. [Table/Fig-4] show the mean CFU count of E.faecalis at two depths (200 μm and 400 μm) at day 1, 3 and 5 for all the medicaments. One-way ANOVA with multiple comparisons (at 200 μm and 400 μm) found statistically significant differences among the groups at various intervals (p=<0.001). [Table/Fig-5] shows a profile plot of estimated marginal means.
Mean values of CFU mL-1 of E.faecalis of intracanal medicament at 200 μm and 400 μm depth, and at three time intervals (days 1, 3 and 5) and ANOVA.
| Groups | 200 μm | 400 μm |
|---|
| Day 1 | Day 3 | Day 5 | Day 1 | Day 3 | Day 5 |
|---|
| Chlorhexidine | 13.166aA | 6.66aB | 1.166aC | 2.00aA | 1.166aB | 0.83aC |
| Neem | 38.166aA | 26.83aA | 22.33aB | 22.66aA | 20.83aA | 15.00aB |
| Calcium hydroxide | 353.33bA | 323.3aA | 206.66aB | 270.00bA | 213.33aA | 160.00aB |
| Aloevera | 700.00c | 700.0b | 700.0b | 700.00c | 700.00b | 700.00b |
| Saline | 1100.00d | 1100.00c | 1100.00c | 1100.00d | 1100.00c | 1100.00c |
| One-way ANOVA test for CFU between groups (200 and 400 μm depth). | F | F |
| 88.862 | 69.024 | 74.167 | 98.269 | 74.012 | 75.946 |
| p-value | p-value |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
CFU - Colony forming Units
The data were statistically analysed with one-way analysis of variance (ANOVA) with multiple comparisons
*Scheffes Post-Hoc test: Different letters indicate significant difference (p< 0.05). Lower-case letters indicate differences in vertical directions. Capital letters indicate differences in horizontal direction
Profile plot of estimated marginal means.
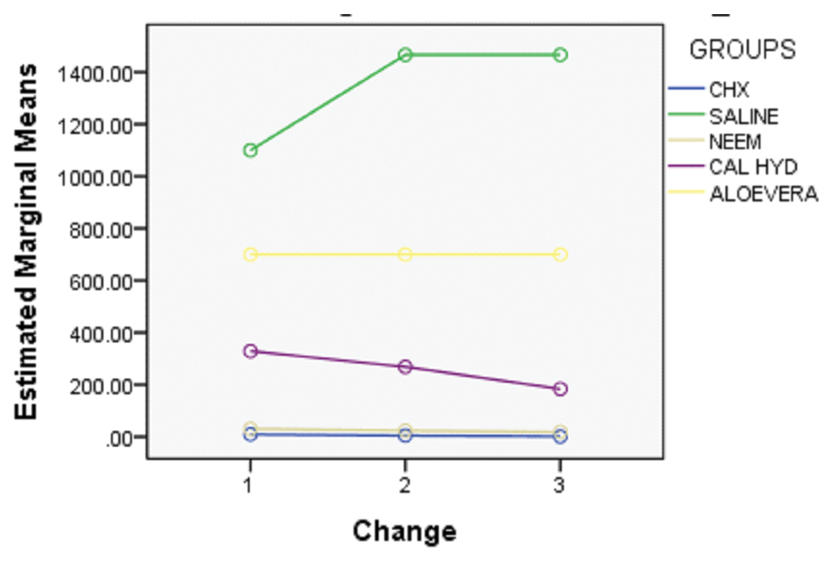
For day 1, at 200 μm and 400 μm depth, no statistical significance were noted in CFU count reduction between CHX and Neem; however significant differences were noted comparing CHX/neem with calcium hydroxide, aloevera and saline. For day 3 and 5, at 200 μm and 400 μm depth, no statistical significance were noted in CFU count reduction between CHX, neem and calcium hydroxide; however, statistical significance was noted between CHX/neem/calcium hydroxide and aloevera/saline. Hence, CHX and neem were most effective in reducing E. faecalis colony count at day 1 (200 μm and 400 μm), followed by CHX, neem and calcium hydroxide at day 3 and 5. Aloevera at both the depths was not as effective as the other medicaments.
Discussion
A major pre-requisite for success of root canal treatment is to disinfect the entire root canal system by eliminating microbes from the root canal system which are the possible sources of infection [17]. Chemomechanical cleaning and shaping of the canal effectively reduces microbial numbers; however, complete disinfection frequently cannot be accomplished. It is generally believed that residual microorganisms can be further reduced by dressing the canal with a intracanal medicament between successive treatment sessions [3,18]. Calcium hydroxide is commonly used for this purpose. However, few studies have questioned the efficacy of calcium hydroxide in reducing microbial numbers even after prolonged contact with the root canal [19].
To study the efficacy of endodontic medications in the disinfection of dentinal tubules, the in vitro model developed by Haapasalo M and Orstavik D has been used [15]. Lynne RE et al., modified this model to involve quantitative analysis of bacteria in the dentinal tubules, to define the percentage reduction in the colony forming units in infected dentin before and after the application of intracanal medicaments [20]. This model has clear limitations because it does not reflect the situation in apical dentin, which is mostly sclerotic [21]. To compensate for the apical sclerosis, the middle third of the canal was selected.
E. faecalis was chosen as the test organism because it is a facultative organism that is non fastidious, easy to grow, efficiently and rapidly colonises tubules [19]. It has been used extensively in endodontic research because it has been found to be present in 63% of teeth with post treatment failures [22]. The inherent ability of E.faecalis to tolerate starvation, high pH, high salt concentration, biofilm formation and resistance to antibiotic has made its eradication most challenging [19,23].
Various intracanal medicaments have been used to minimise or eradicate E. faecalis from root canal space but have not been very efficient [17,24,25]. Few studies have compared the effectiveness of irrigants with agitation technique in elimination of E. faecalis in root canal space [26,27]. Various in vitro and in vivo studies have tested herbal extracts for better antimicrobial activity and biocompatibility [10,24,28]. The present study compared chlorhexidine, neem, aloevera and calcium hydroxide. The plant extraction was done by soxhlet technique and to the knowledge of the authors no previous studies have been done using this method.
Studies comparing 2% CHX gel and 2% CHX liquid found elimination of Staphylococcus aureus and Candida albicans within 15 seconds, whereas the gel formulation killed E. faecalis within 1 minute [7,29]. In the present study, 2% CHX liquid provided almost 100% inhibition of E. faecalis at a depth of 200 μm as well as 400 μm from day 1 to day 5. The plausible reason could be the bactericidal dosage of 2% CHX, increased diffusion of the medicament into the dentinal tubules and substantivity. The results of this study are in agreement with previous studies [17,18]. Portenier J et al., found that dentin matrix and type I collagen have inhibitory effect on chlorhexidine, but the study tested a concentration of 0.2% CHX. The inhibitory effect of dentin on CHX can be overcome by increase in concentration (2%) as used in this study [30].
In the present study, neem leaf extract was highly efficient in reducing E. faecalis within the root canals at both depths (200 μm and 400 μm) and at all time intervals with statistically significant difference when compared to aloevera and calcium hydroxide. This is in agreement with previous studies [31,32]. However, few studies have reported leaf extract of neem is very effective against S. mutans and S. aureus, but ineffective on E. faecalis at lower concentration [33,34]. The possible reason could be the use of bark or the technique of extraction. Bohara A et al., concluded that neem leaf extract exhibited significant antibacterial effect against E. faecalis which is in accordance to our study [32]. Neem contains various active phytoconstituents such as alkaloids, glycosides, trepenoids, steroids and tannins which have been found to be effective in the management of various oral conditions, thus exhibiting its biocompatibility [35,36].
In the present study, the antimicrobial effect of Ca(OH)2 against E. faecalis was lower than CHX and neem and was statistically significant. This could possibly be due to the bacterial tolerance of pH changes by activation of specific proton pumps, specific enzymes and/or buffering mechanisms, which help bacteria to neutralise the environmental pH. This relative inefficacy of Ca(OH)2 against E. faecalis was in accordance with previous studies [5,8,25].
Aloevera group was least effective against E. faecalis at all time intervals (day 1,3 and 5) and at depths of 200 μm and 400 μm when compared to CHX and neem with statistical significance. This result is in accordance with that given by Valera MC et al., [37]. However, Bhardwaj A et al., showed that aloevera exhibited approximately 78% and 80% inhibition at depths of 200 μm and 400 μm respectively from day 1 to 5 [38]. Ehasani M et al., in a study concluded that aloevera had mild antimicrobial effect against E. faecalis [39]. Kurian B et al., compared the antimicrobial efficacy of extracts of mushroom, aloevera leaves and calcium hydroxide against E. faecalis and found that the percentage reduction of CFUs was highest in mushroom followed by aloevera and calcium hydroxide [40]. They attributed that the poor antimicrobial action of aloevera was due to its lesser penetration into the dentinal tubule as a result of higher molecular weight (1000 kDa).
Limitation
The limitation of the present study was that: i) it was an in vitro study and accurate replication of clinical conditions were not achieved; (ii) it was not possible to directly compare studies due to difference in the methodology and technique of plant extraction used in the study. The present study reinforces the literature regarding the efficacy of neem as a better herbal irrigant compared to aloevera. Further studies are required to corroborate the results of present study.
Conclusion
Within the limitations of this study, it can be concluded that; 2% chlorhexidine was the best intracanal medicament which consistently reduced colony forming unit count of E. faecalis at days 1, 3 and 5, at both depths of 200 μm and 400 μm. Neem showed similar activity compared to chlorhexidine, and better activity than calcium hydroxide and aloevera.
The use of aloevera as herbal intracanal medicament against E. faecalis is questionable.
CFU - Colony forming Units
The data were statistically analysed with one-way analysis of variance (ANOVA) with multiple comparisons
*Scheffes Post-Hoc test: Different letters indicate significant difference (p< 0.05). Lower-case letters indicate differences in vertical directions. Capital letters indicate differences in horizontal direction