Atopic Dermatitis (AD) or eczema is a chronic and relapsing, itchy skin inflammatory condition in infancy and childhood. The diagnosis is based on pruritus and an eczematous dermatitis with typical presentation. A five-month-old infant who was suffering from itches and eczema since three months, was diagnosed with AD. After clinical diagnosis of AD, the condition seemed to be superimposed by a bacterial infection, an empirical treatment of antibiotic (oral cephalexin) was started. However, the patient showed no improvement. As per the hospital protocol, he received clindamycin, to which the lesions responded and totally resolved. The AD in our case was reported due to severe facial impetigo with an unusual feature that it resembled a primary immunodeficiency. However, in our case, the patient had an intact immune system and did not have any transient hypogammaglobulinemia.
Clindamycin, Hypogammaglobulinemia, Immunodeficiency, Infant, Wiskott-aldrich syndrome
Case Report
The patient was a five-month-old boy with itchy, scaly and erythematosus of the skin that started first on his face and limb extensors. He was diagnosed with AD and had to use emollient (vaseline) and topical steroid (hydrocortisone) daily, since he was two months old.
When he was four-month-old, he was hospitalised with an increased intensity and frequency of skin lesions as they had started appearing as crusted vesicles. In the outpatient department, cephalexin (50 mg/kg/day divided into q6h) was prescribed and used but no signs of recovery were seen. Ten days before hospitalisation, yellow crusts began to cover half of his face including the forehead and forehead-parietal area (sizing up to 20 cm) as plaque. Thus, he was diagnosed with impetigo and admitted to the hospital for immunodeficiency tests.
Physical examination indicated an infant with normal vital signs (except for a fever). During his first visit, symptoms included yellow, scaly, plaque-like lesions covering the right ear, forehead, and half of his head [Table/Fig-1].
Yellow, scaly, plaque-like lesions covering the right ear, forehead and half of his head.

The eyes and the left ear appeared to be normal. Dry skin and desquamation were noticed around extensors. Several tests were done during hospitalisation including staining, skin-lesion culture, Complete Blood Count (CBC) with differential, blood culture, serum immunoglobulin levels, Tzanck smear of the lesions for Herpes simplex, and Erythrocyte Sedimentation Rate (ESR). Staining and skin-lesion culture indicated methicillin Resistant Staphylococcusaureus (MRSA) sensitive to lincomycin, vancomycin and clindamycin, ciprofloxacin, and erythromycin; resistant to cloxacillin. The Tzanck smear was negative. All the tests were in normal range upon examination.
ESR and CRP were normal and [Table/Fig-2] shows the rest of the results. Clindamycin IV (30mg/kg/day divided into q8h), topical steroids (hydrocortisone) daily and topical antiseptic (mupirocin, divided into q8h) were prescribed but cephalosporin was not used because the patient had not responded to them in earlier treatments and the bacteria culture was also cloxacillin-resistant. The fever reduce the second day and some degree of recovery was observed on the fourth day after hospitalisation [Table/Fig-3].
Laboratory investigation.
| Immunoglobulin | Immunoglobulin Level | CBC Differential | Count |
|---|
| IgE (ELISA) | 350 iu/mL (Nomal: 3-12 Month <37) | WBC (×103/μL) | 11.2 |
| IgA | 0.96 g/L (Nomal: 0.13-1.02) | Eosinophil (%) | 12 |
| IgM | 0.81 g/L (Nomal: 0.81-1.45) | Neutrophil (%) | 15 |
| IgG | 7/11 g/L (Nomal: 1/8-9) | Lymphocyte (%)Monocyte (%)Haemoglobin (g/dL)Platelet (×103/μL) | 76610706 |
Recovery observed on the fourth day after hospitalisation.

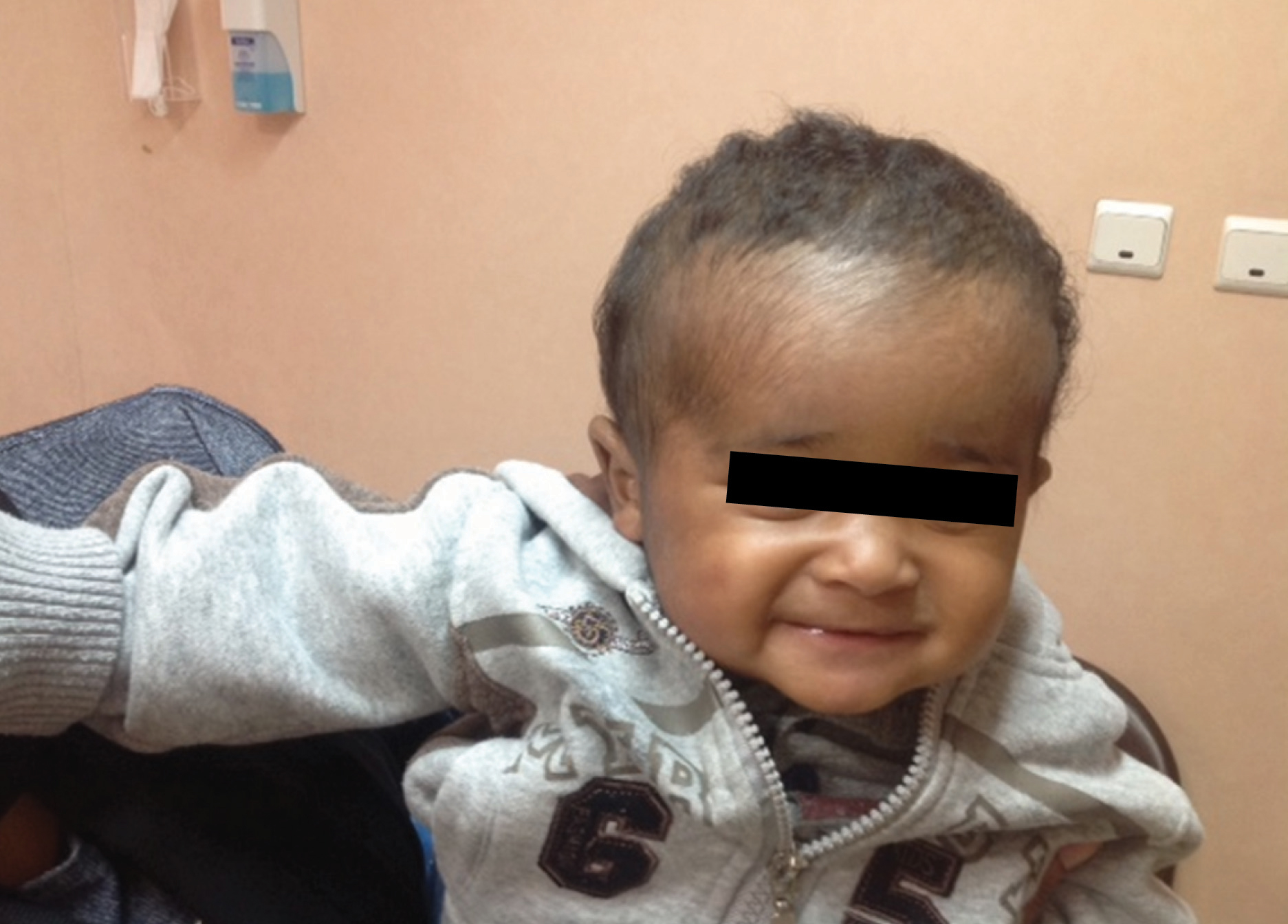
During the hospital course, intravenous clindamycin was prescribed for one week and then the patient was discharged on erythromycin syrup (50 mg/kg/day) for one week. A month later, the patient was examined at the clinic and all his lesions had disappeared and he recovered completely [Table/Fig-4].
A month later, the patient recovered completely.
Discussion
Atopic Dermatitis or eczema is the most common skin disease during infancy and childhood. Between 10%-30% of all children and 1%-3% of all adults with food allergy or a family history of other atopic diseases (e.g., asthma and allergic rhinitis) are affected [1,2]. It is a genetic disorder that emerges as a malfunctioning of the skin’s protective epidermis barrier. This can be attributed to the lack of antimicrobial peptides required by the host’s immune system. It might also occur because of the decreased responsiveness of the innate immune system and the increased responsiveness of the adaptive immune system (including T lymphocytes) to allergens and microbes. Eventually, it emerges as chronic inflammations and skin infections [1,3,4]. There are two types of AD namely, atopic eczema and non-atopic eczema. An increased immunoglobulin E level in atopic patients makes it IgE-mediated. Between 70%-80% of AD cases are of this type. Nevertheless, the non-atopic eczema constituting the remaining 20%-30% of cases are not IgE-mediated. In both types, eosinophils-levels increase, however, TH2-dependent cytokines (IL-13, IL-4) only increase in patients with atopic eczema [1].
Atopic Dermatitis diagnosis is based on chronic and recurring eczema and itchy skin [5]. The hallmark of AD is itchy eczema in various skin areas which might be different, depending on the patient’s age. During infancy, AD is most commonly seen on the face and lower limb extensors. In older children, it is mostly seen in flexor areas and in adults, it is usually not associated with a specific area [6]. Based on the intensity, AD could be divided into three: mild, moderate and severe forms. The mild form does not emerge as frequent itches and the skin is dry with no erythematosus. The moderate form frequently itches with no skin lesions. The severe form shows dry, crusted, oozing skin with increased itchiness [1,5]. Severe, frequent itchiness which results in skin cracks and genetic disorders and can put the patients at risk of viral and bacterial infections. Staphylococcus aureus bacteria colonize the skin and cause impetigo. Viral (e.g., Herpes simplex) or fungal (e.g., dermatophytes) skin infections might also be observed in the patients [7].
Impetigo is an eczema observed in two bullous and non-bullous forms [8]. Staphylococcus aureus bacteria and Group A Beta-Haemolytic Streptococcus (GABHS) are the most common causes of impetigo [9]. Atopic Dermatitis treatment involves emollients to moisturize the skin and topical steroids to suppress the inflammation. Other medications (e.g., antibiotics, antihistamines, oral steroids, and immunosuppressant drugs) are prescribed in special cases based on the patient’s condition [10].
The aim of the present case was to report an unusual case of intensive impetigo which is rarely seen in healthy individuals that show no signs of immunodeficiency. The infection occurred as eczema resembling primary immunodeficiency diseases (e.g., Wiskott-Aldrich syndrome) during differential diagnosis [6]. The examinations, however, showed no primary immunodeficiency disease. Furthermore, even hypogammaglobulinemia, which is reported in some AD patients, was not noted [2]. Wiskott-Aldrich syndrome or other immunodeficiency diseases such as Omenn syndrome or Hyper-IgE syndrome which were considered as the differential diagnosis were not observed either. Although, some studies have reported post-AD transient hypogammaglobulinemia, the patient did not show any symptoms for the same. The Staphylococcusaureus bacteria play an important role in intensifying AD symptoms. The bacteria worsen the disease by producing super antigens and interleukins. They also cause severe infection by reproducing T lymphocytes [11]. MRSA was seen in the patient’s culture and smear. Other observations included mild leukocytosis, increases in serum IgE (350), increase eosinophils levels (12%) in peripheral blood smear, and normal serum IgG levels. Viral infections (e.g., Herpes simplex) may worsen AD, but the negative Tzanck smear results of our case indicated no virus. The most common cause of soft tissue infection in AD patients is Staphylococcusaureus. In AD patients due to malfunction of skin barrier and because these patients may have multiple referring to health care units or recurrent hospital admission, so that they predispose to colonization of Staphylococcus aureus and specially to MRSA, consequently we should consider MRSA in severe soft tissue infection of these patients and therefore, commence clindamycin or vancomycin, as empirical therapy in these patients.
Impetigo infections should be studied since they are the most common among AD infants. Once the cause is identified, it can be removed [12]. Due to the high immunoglobulin E levels (350), the disease was diagnosed as an IgE-mediated eczema which is the most common type of AD in 70% to 80% of the cases.
Conclusion
The present report was carried out for three main reasons. Firstly, it was a case of intensive impetigo rarely seen in individuals with any immunodeficiency. Secondly, the infection occurred as an eczema which happened to be similar to primary immunodeficiency diseases (e.g., Wiskott-Aldrich syndrome) however, our case showed no primary immunodeficiency disease. Third, even hypogammaglobulinemia, which is reported in some AD patients, was not noted in our case. Although, no recovery signs were observed after taking oral antibiotics, the patient immediately responded to antibiotic injections. It is important to note that in our case, cooperation, patient care and successful treatment, prevented the recurrence during a one year follow up period.
[1]. Kanchongkittiphon W, Gaffin JM, Phipatanakul W, Child with atopic dermatitisAnnals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 2015 114(1):6-11.10.1016/j.anai.2014.08.01625528736 [Google Scholar] [CrossRef] [PubMed]
[2]. Yasutomi M, Okazaki S, Kawakita A, Hayashi H, Murai H, Mayumi M, Case of atopic dermatitis in infant treated with Chinese herbal medicines and nsaids ointment, which induced weight loss, electrolyte disturbance and hypoproteinemia]Arerugi = (Allergy) 2013 62(7):827-32. [Google Scholar]
[3]. Zeeuwen PL, Kleerebezem M, Timmerman HM, Schalkwijk J, Microbiome and skin diseasesCurrent opinion in allergy and clinical immunology 2013 13(5):514-20.10.1097/ACI.0b013e328364ebeb23974680 [Google Scholar] [CrossRef] [PubMed]
[4]. Alomar A, Can microbial superantigens influence atopic dermatitis flares?Chemical immunology and allergy 2012 96:73-76.10.1159/00033188722433373 [Google Scholar] [CrossRef] [PubMed]
[5]. Katayama I, Kohno Y, Akiyama K, Aihara M, Kondo N, Saeki H, Japanese Guideline for Atopic Dermatitis 2014Allergology international : official journal of the Japanese Society of Allergology 2014 63(3):377-98.10.2332/allergolint.14-RAI-0769 [Google Scholar] [CrossRef]
[6]. Lio PA, Lee M, LeBovidge J, Timmons KG, Schneider L, Clinical management of atopic dermatitis: practical highlights and updates from the atopic dermatitis practice parameter 2012The Journal of Allergy and clinical immunology In practice 2014 2(4):361-69.10.1016/j.jaip.2014.02.01525017522 [Google Scholar] [CrossRef] [PubMed]
[7]. O’Sullivan C, Baker MG, Serious skin infections in children: a review of admissions to Gisborne Hospital (2006-2007)The New Zealand Medical Journal 2012 125(1351):55-69. [Google Scholar]
[8]. Salah LA, Faergemann J, A retrospective analysis of skin bacterial colonisation, susceptibility and resistance in atopic dermatitis and impetigo patientsActa Dermato-Venereologica 2015 95(5):532-35.10.2340/00015555-199625367860 [Google Scholar] [CrossRef] [PubMed]
[9]. Hartman-Adams H, Banvard C, Juckett G, Impetigo: diagnosis and treatmentAmerican Family Physician 2014 90(4):229-35. [Google Scholar]
[10]. McAleer MA, Flohr C, Irvine AD, Management of difficult and severe eczema in childhoodBMJ 2012 345:e477010.1136/bmj.e477022826585 [Google Scholar] [CrossRef] [PubMed]
[11]. Wollenberg A, Feichtner K, Atopic dermatitis and skin allergies-update and outlookAllergy 2013 68(12):1509-19.10.1111/all.1232424410780 [Google Scholar] [CrossRef] [PubMed]
[12]. Sugiyama A, Nishie H, Masumoto N, Murakami Y, Furue M, Treatment of facial lesion in infantile and childhood atopic dermatitisJapanese Journal of Allergology61(2):204-214. [Google Scholar]