Cutaneous Ischaemia Following Terlipressin Therapy for Hepatorenal Syndrome
Aneesh Basheer1, Dayanandan Yoganandan2, Meera Baby John3, Prem Kumar Guna4
1 Associate Professor, Department of General Medicine, Pondicherry Institute of Medical Sciences, Puducherry, India.
2 Senior Resident, Department of General Medicine, Pondicherry Institute of Medical Sciences, Puducherry, India.
3 Junior Resident, Department of General Medicine, Pondicherry Institute of Medical Sciences, Puducherry, India.
4 Assistant Professor, Department of General Medicine, Pondicherry Institute of Medical Sciences, Puducherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Aneesh Basheer, Associate Professor, Department of General Medicine, Pondicherry Institute of Medical Sciences, Kalapet-605014, Puducherry, India.
E-mail: basheeraneesh@gmail.com
Hepatorenal Syndrome (HRS) is a potentially fatal complication of hepatic failure and portal hypertension. Among the various treatments available including octreotide, midodrine and albumin, the current standard of care for stable patients is terlipressin along with albumin. Although, terlipressin is considered safer than its parent molecule vasopressin, there have been reports of ischaemic side effects related to vasoconstrictor effects such as myocardial and mesenteric ischaemia. Cutaneous ischaemia has been reported in as few as 20 cases to date. We report the case of a gentleman who developed ischaemic skin lesions due to peripheral vasoconstriction following terlipressin thereapy for HRS and rapid reversal on discontinuing the drug. This and other similar cases highlight the need for larger studies exploring ischaemic side effects of terlipressin as well as close monitoring of patients for early detection of this side effect.
End stage liver disease, Splanchnic circulation, Vasoconstriction, Vasopressin
Case Report
A 49-year-old male, diagnosed with chronic liver disease and portal hypertension secondary to Non Alcoholic Fatty Liver Disease (NAFLD) was admitted for abdominal pain, worsening abdominal distension and breathlessness for 10 days. There was no history of fever or altered sensorium. On examination he was conscious and co operative with pitting pedal oedema and icterus. Blood pressure was 94/60 mmHg and pulse rate was 88/minute. Abdomen was non tender, distended and positive for shifting dullness. Apart from gynaecomastia and spider angiomas there were no other stigmata of liver cell failure. Other systemic examination were unremarkable.
Laboratory workup revealed leucocytosis with neutrophilic predominance and thrombocytopenia [Table/Fig-1]. Renal functions tests were normal. Liver functions were deranged. His prothrombin time was prolonged with an International Normalisation Ratio (INR) of 2.4. Blood and urine cultures were sterile. Urine microscopy was normal. There was mild hyponatremia. Ascitic fluid analysis revealed high gradient suggestive of portal hypertension (SAAG of 1.4).
Laboratory parameters of the patient at baseline.
| Parameter | Patient’s value | Reference range |
|---|
| Haemoglobin (gm/dL) | 9.1 | 13.3-16.2 |
| Total white cell count (cells/cu.mm) | 25,800 | 4000-11,000 |
| Differential count | N-90%; L-10% | N-40 to 70%; L-20 to 50% |
| Platelet count (cells/cu.mm) | 94,000 | 1,50,000-4,00,000 |
| Blood urea (mg/dL) | 24 | 20-40 |
| Serum creatinine (mg/dL) | 0.9 | 0.7-1.1 |
| Bilirubin (total) (mg/dL) | 6.6 | 0.3-1.3 |
| Bilirubin (direct) (mg/dL) | 1.7 | 0.1-0.4 |
| Alanine aminotransaminase (ALT) (U/L) | 37 | 7-41 |
| Aspartate aminotransferase (AST) (U/L) | 55 | 12-38 |
| Alkaline Phosphatase (U/L) | 86 | 33-96 |
| Serum total protein (gm/dL) | 6.1 | 6.7-8.6 |
| Serum albumin (gm/dL) | 2.5 | 3.5-5.5 |
| INR | 2.4 | |
| Serum sodium (mEq/L) | 127 | 135-145 |
| Serum potassium (mEq/L) | 3.8 | 3.5-5.5 |
| Urine WBCs (cells/hpf) | Nil | 0-2 |
| Urine RBCs (cells/hpf) | Nil | 0-2 |
| Urine albumin | trace | Nil |
| Urine sugar | Nil | Nil |
| Blood culture | Sterile | Sterile |
| Urine culture | Sterile | Sterile |
| Ascitic fluid cell count (cells/cu.mm) | 40 | - |
| Ascitic fluid albumin (gm/dL) | 1.1 | - |
| Ascitic fluid culture | Sterile | Sterile |
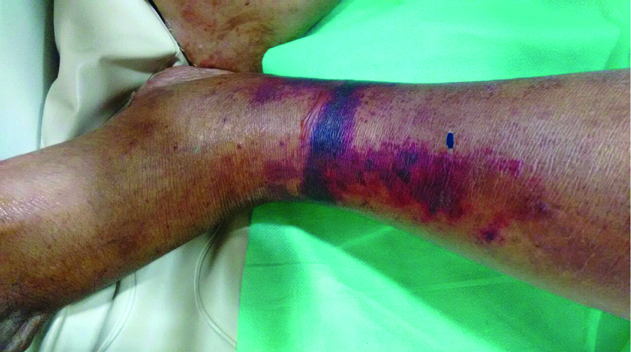
Initial working diagnosis was spontaneous bacterial peritonitis complicating chronic liver disease with portal hypertension (Child Pugh class C with a score of 11). However, absence of fever and a bland relatively acellular ascitic fluid made this unlikely. Sepsis with undetermined focus was highly likely and hence following blood and urine cultures (which were later found to be sterile) patient was started on broad spectrum antibiotic (piperacillin-tazobactam). While his abdominal pain and distension improved with this treatment, his urine output showed a decline after three days. Renal functions deteriorated (blood urea-106 mg/dL, serum creatinine-2.9 mg/dL). In view of underlying portal hypertension, a diagnosis of HRS was made. Urine spot sodium was 14 meq/L and urine protein was undetectable by dipstick. Ultrasonography of kidneys was normal. He was started on intravenous albumin and vitamin K. Injection terlipressin was started at a dose of 1 mg every six hours. There was improvement in urine output and renal function after 24 hours. However, on the third day of terlipressin, patient complained of pain in the legs. He developed bluish discolouration of toes and fingers, progressing to blackish discolouration of skin over legs [Table/Fig-2]. The extremities were cold and bilateral dorsalis pedis pulses were feeble. Doppler study of lower limbs showed diminished arterial flow distally. Although, terlipressin is believed to act specifically at the splanchnic vessels, the temporal association of signs and symptoms was compelling, and a literature search revealed that similar adverse events have been reported following Terlipressin use albeit rarely [1-3]. Terlipressin was promptly discontinued and over the next 48 hours the patient improved dramatically with disappearance of pain and return of warmth in the extremities. Repeat Doppler after three days showed normal flow pattern while skin discolouration disappeared after a week [Table/Fig-3].
Blackish discolouration of skin over leg (left side shown here) on the third day of administration of terlipressin.
Resolving skin discolouration over leg after a week of discontinuing Terlipressin.
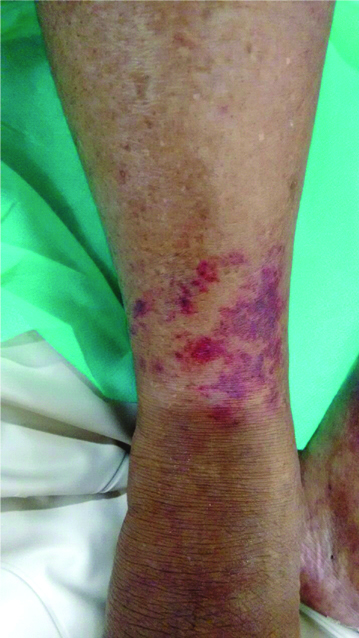
The sequence of events suggests that our patient developed peripheral vasoconstriction following terlipressin therapy leading to acrocyanosis, ischaemia of distal skin and pregangrenous changes, promptly reversed by discontinuation of the drug.
Discussion
Hepatorenal syndrome is a serious complication of cirrhosis with portal hypertension. Despite poor prognosis, intravenous albumin and octreotide have been used for treatment with limited success. Terlipressin, a vasopressin analogue has emerged as standard of care due to specific action on splanchnic vasculature; hence the assumption that systemic side effects are low.
HRS has traditionally been classified as type 1 and 2, the former being quite aggressive. The diagnosis of HRS itself is quite complex as acute kidney injury in a patient with hepatic failure and portal hypertension is often multifactorial. Acute rise in serum creatinine by more than 0.3 mg/dL within 48 hours (consistent with the KDIGO criteria) often with oliguria along with absence of shock, nephrotoxic drug exposure and obstructive uropathy or parenchymal kidney diseases (by ultrasonography) makes HRS highly likely in the setting of liver disease with portal hypertension. Our patient probably developed type 1 HRS since he had a rapid worsening of renal function (doubling of serum creatinine within three days and oliguria) and arose on a background of underlying sepsis (that generally precipitates type 1 HRS). Terlipressin along with albumin infusion has become standard therapy for HRS in most countries (not available in the United States). The standard dose is 1-2 mg given as boluses every 4 to 6 hours. Its effects in HRS have been studied in randomised controlled trials. One landmark trial that randomised patients to terlipressin or midodrine with octreotide found that more patients in the terlipressin group attained a normal creatine [4]. Moreover, terlipressin has become recommended therapy with albumin for HRS patients, thanks to the data from meta-analysis of several trials comparing it with placebo or albumin alone, showing high reversal rates and reduced mortality [5].
Adverse events with terlipressin are relatively mild including headache, pale skin and abdominal cramps. Hypertension and bradycardia are well known side effects. Hyponatremia occurs in some patients [6]. Side effects related to vasoconstriction like myocardial infarction, mesenteric ischaemia and skin necrosis are rare with incidence below 5% [7]. Only 20 cases of skin ischaemia secondary to terlipressin have been reported as of 2014. In these cases, latency between terlipressin initiation and development of skin manifestation was around three days, as in our patient. About 50% of cases had alcoholic cirrhosis as the underlying liver disease; another 25% had NAFLD and obesity. Based on this data, Ozel Coskun BD et al., concluded that patients with alcoholic cirrhosis may be at higher risk for terlipressin induced ischaemia [1]. However, this preponderance of alcoholic cirrhosis as the underlying disease in these cases might just be a reflection of this being the most common cause for cirrhosis worldwide. Other case reports suggest that obesity, concurrent vasopressors, pre-existing ischaemic disease and spontaneous bacterial peritonitis may predispose to ischemic skin complications [2,8]. Subsequently in 2016, two more cases of skin necrosis have been reported secondary to terlipressin use, one for upper digestive bleed and other for HRS. In both cases, the complication developed within 48 hours of administration and was irreversible even after withdrawal of the drug [3].
Although, terlipressin is believed to have a better side effect profile than vasopressin, recent evidence suggests otherwise. A meta-analysis failed to show a significant difference between vasopressin and terlipressin in terms of adverse events [9]. Since cutaneous ischaemia is related to vasoconstrictor properties, it is likely to be reversible if detected well in advance. Rapid reversal of cutaneous signs and symptoms in our case indicates the same. In the report by Ozel Coskun BD et al., skin lesions progressed to necrosis necessitating debridement and skin grafting, despite cessation of terlipressin. Ischaemic heart disease and atherosclerotic changes in upper limb vessels in that patient probably propagated skin ischaemia terminating in necrosis [1]. Another meta-analysis found significant reversal of HRS with terlipressin, but also reported a higher rate of ischemic events with terlipressin compared to a control [10]. Reversal of ischaemic adverse events after cessation or dose reduction has been shown in another meta-analysis [11]. Besides, there is now some compelling evidence comparing terlipressin and nor epinephrine for HRS, reporting lower adverse events with nor epinephrine [12].
Conclusion
Our case, and available evidence suggests that cutaneous ischaemia associated with terlipressin may not be uncommon. Patients with ischaemic disease of heart or vessels, use of other vasopressors, obesity and spontaneous bacterial peritonitis need close monitoring for early detection. Prompt discontinuation of the drug may be life-saving and limb-saving. Larger studies exploring ischaemic side effects of terlipressin may shed more light in this aspect.
[1]. Ozel Coskun BD, Karaman A, Gorkem H, Buğday I, Poyrazoğlu OK, Senel F, Terlipressin-induced ischemic skin necrosis: a rare associationAm J Case Rep 2014 15:476-79.10.12659/AJCR.89108425360696 [Google Scholar] [CrossRef] [PubMed]
[2]. Yefet E, Gershovich M, Farber E, Soboh S, Extensive epidermal necrosis due to terlipressinIsr Med Assoc J 2011 13:180-81. [Google Scholar]
[3]. Cleva RD, Santarém OLDA, Herman P, Terlipressin-induced irreversible severe skin necrosis: a dreadful complicationAnn Clin Case Rep 2016 1:1040 [Google Scholar]
[4]. Cavallin M, Kamath PS, Merli M, Fasolato S, Toniutto P, Salerno F, Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trialHepatology 2015 62(2):567-74.10.1002/hep.2770925644760 [Google Scholar] [CrossRef] [PubMed]
[5]. Gluud LL, Christensen K, Christensen E, Krag A, Terlipressin for hepatorenal syndromeCochrane Database Syst Rev 2012 9:CD00516210.1002/14651858.CD005162.pub3 [Google Scholar] [CrossRef]
[6]. Solà E, Lens S, Guevara M, Martín-Llahí M, Fagundes C, Pereira G, Hyponatremia in patients treated with terlipressin for severe gastrointestinal bleeding due to portal hypertensionHepatology 2010 5:1783-90.10.1002/hep.2389320931555 [Google Scholar] [CrossRef] [PubMed]
[7]. Uriz J, Gines P, Cárdenas A, Sort P, Jimenez W, Salmeron JM, Terlipressin plus albumin infusion: an effective and safe therapy of hepatorenal syndromeJ Hepatol 2000 33:43-48.10.1016/S0168-8278(00)80158-0 [Google Scholar] [CrossRef]
[8]. Vaccaro F, Giorgi A, Riggio O, De Santis A, Laviano A, Rossi-Fanelli F, Is spontaneous bacterial peritonitis an inducer of vasopressin analogue side-effects? a case reportDig Liver Dis 2003 35:503-06.10.1016/S1590-8658(03)00225-1 [Google Scholar] [CrossRef]
[9]. Polito A, Parisini E, Ricci Z, Picardo S, Annane D, Vasopressin for treatment of vasodilatory shock: an ESICM systematic review and meta-analysisIntensive Care Med 2012 38(1):910.1007/s00134-011-2407-x22127480 [Google Scholar] [CrossRef] [PubMed]
[10]. Fabrizi F, Dixit V, Messa P, Martin P, Terlipressin for hepatorenal syndrome: a meta-analysis of randomized trialsInt J Artif Organs 2009 32(3):133-40.10.1177/03913988090320030319440988 [Google Scholar] [CrossRef] [PubMed]
[11]. Fabrizi F, Dixit V, Martin P, Meta-analysis: terlipressin therapy for the hepatorenal syndromeAliment Pharmacol Ther 2006 24(6):935-44.10.1111/j.1365-2036.2006.03086.x16948805 [Google Scholar] [CrossRef] [PubMed]
[12]. Nassar Junior AP, Farias AQ, D’ Albuquerque LA, Carrilho FJ, Malbouisson LM, Terlipressin versus norepinephrine in the treatment of hepatorenal syndrome: a systematic review and meta-analysisPLoS One 2014 9(9):e10746610.1371/journal.pone.010746625203311 [Google Scholar] [CrossRef] [PubMed]