Hemangiopericytoma: A Rare Mass Arising in the Kidney
Gregory Pathrose1, Anuj Deep Dangi2, Mayank Gupta3, Ramani Manoj Kumar4, Nitin Sudhakar Kekre5
1 Postgraduate Registrar, Department of Urology, Christian Medical College, Vellore, Tamil Nadu, India.
2 Associate Professor, Department of Urology, Christian Medical College, Vellore, Tamil Nadu, India.
3 Assistant Professor, Department of Pathology, Christian Medical College, Vellore, Tamil Nadu, India.
4 Professor, Department of Pathology, Christian Medical College, Vellore, Tamil Nadu, India.
5 Professor, Department of Urology, Christian Medical College, Vellore, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Gregory Pathrose, House No. XX/156, Hill Road, Alwaye-683101, Kerala, India.
E-mail: greglycan@gmail.com
Hemangiopericytoma, a tumour arising from pericytes is an unusually rare kidney neoplasm. It is difficult to establish a preoperative diagnosis. Here, we present a case report of a 47-year-old gentleman who presented with haematuria and underwent left radical nephrectomy as preoperative imaging was suggestive of Renal Cell Carcinoma (RCC). The final histopathological diagnosis was hemangiopericytoma of the left kidney.
Cytokeratin, Desmin, Pericyte
Case Report
A 47-year-old nonsmoker, hypertensive presented with painless gross haematuria for one week. He presented with haematuria in 2011, which resolved without active intervention. His general and physical examination were normal. His haemogram, urine cytology, blood chemistry and chest X-ray were within normal limits.
Contrast Enhanced Computed Tomography (CECT) of the abdomen revealed 14×10×13 cm large, lobulated, well defined mildly enhancing soft tissue density lesion (48-50 HU) with areas of necrosis involving the mid and the lower region of the left kidney [Table/Fig-1].
CECT showing large hilar mass arising from middle and lower pole splaying the renal pelvis and abutting the psoas muscle.
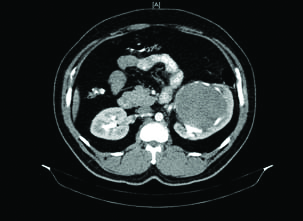
The lesion was splaying and distorting the renal pelvis. The neoplasm was abutting the left psoas and Gerota’s fascia. The renal vein was compressed but was not infiltrated. There was no significant regional lymph node enlargement. The differential diagnosis of complex Bosniak cyst (type III-IV), cystic RCC and RCC with atypical features were suggested.
The patient underwent left laparoscopic radical nephrectomy which had to be converted to open radical nephrectomy due to dense hilar dissection. The postoperative period was uneventful.
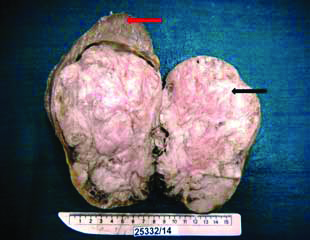
The left radical nephrectomy specimen weighed 950 gm and measured 18×11×9 cm. The kidney appeared enlarged grossly with external lobulations [Table/Fig-2].
Lobulated homogenous grey white cut surface of tumour (black arrow). Red arrow shows normal renal parenchyma.
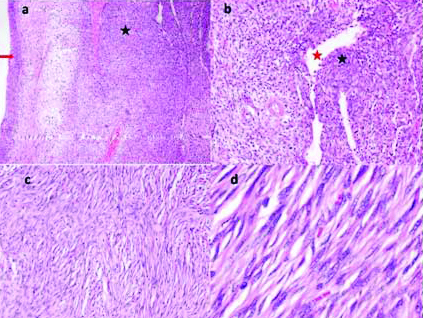
On histopathology tumour was seen reaching the capsule but there was no evidence of breach, vessel emboli or lymph vascular invasion. Renal parenchyma was seen with a circumscribed tumour arranged in fascicles and bundles and few areas displaying storiform arrangement composed of spindle cells displaying elongated nuclei, fine chromatin, inconspicuous nucleoli and eosinophilic cytoplasm [Table/Fig-3a]. In areas, round to fusiform cells displaying bland nuclear chromatin and indistinct cytoplasmic borders along with rich vascular pattern consisting of large and small caliber vessels lined by flattened endothelial cells are evident [Table/Fig-3b]. Perivascular hyalinisation was evident. Myxoid change with occasional mitotic figures was seen in few areas. Necrosis was absent. Tumour reached up to the capsule without extracapsular extension. Tumour involved the wall of renal pelvis. Interspersed lymphocytes and plasma cells were also seen [Table/Fig-3c]. In some areas presence of bland chromatin in tumour cells were seen along with usual features of elongated nuclei [Table/Fig-3d].
a) Tumour (marked with black star) with prominent blood vessels. urothelium is marked by red arrow, (H&E, 4X); b) Round to elongated tumour cells (marked with black star) with small and large blood vessels (red star highlights lumen of a blood vessel), (H&E, 10X); c) Tumour arranged in fascicles with interspersed few small lymphocytes (H&E, 10X); d) Tumour cells display elongated nuclei, bland chromatin and eosinophilic cytoplasm (H&E, 40X).
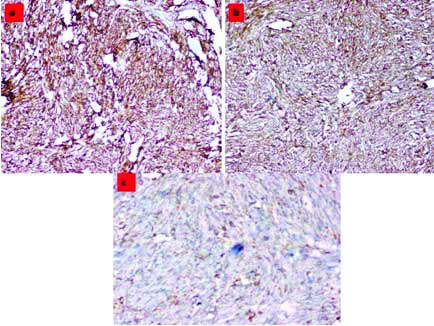
Immunohistochemistry
Spindle cells were diffusely and strongly positive for CD34 [Table/Fig-4a] and CD99 [Table/Fig-4b]. They were positive for bcl-2 [Table/Fig-4c]. CD31 was positive in few rounds to fusiform cells. Transducin-Like Enhancer of split-1 (TLE-1) was focally positive. Desmin, Smooth Muscle Actin (SMA), S-100 and cytokeratin were negative.
Immunohistochemistry: a) Tumour cells diffusely staining for CD34, (10X); b) Tumour cells diffusely staining for CD99 (10X); c) Tumour cells staining for bcl-2 (20X).
Discussion
Hemangiopericytoma is an extremely rare tumour arising from pericytes which are cells surrounding capillaries and help in regulating blood flow through them. Till 2015, only 43 cases have been reported [1]. The description of pericytes was first given by Zimmermann in 1923 [2]. Though found commonly in the head and neck region meninges and pelvis, Hemagiopericytomas are rarely found in the genitourinary system. The term was first coined by Stuart and Murray to describe vascular tumours arising from the pericyte capillaries [2]. These are well circumscribed tumours found in younger age group 40.3 (18-46 years). The male:female ratio of 1.1:1 with first case of hemangiopericytoma was first published by Black and Heinemann in 1942 [3].
These tumours do not have any specific signs and symptoms and grow up to large sizes before they are detected clinically. The main symptoms associated with hemangiopericytomas are haematuria, hypertension and hypoglycaemia. Haematuria is a sign of late presentation with tumour involving the pelvicalyceal system [1]. Hypertension is due to production of rennin by tumour and regressis after the tumour is excised [2]. Hypoglycaemia is due to increased metabolism of glucose within the tumours [2].
As there are no specific radiological features that aid in the preoperative diagnosis of hemangiopericytomas, the diagnosis is usually of exclusion or on biopsy. In the arterial phase of CECT scans, large vessels seem to surround the tumours with well demarcated tumour and displacement of main arteries [4].
On examination, these tumours are solid with minimal extension into perinephric structures. The tumours that resemble hemangiopericytoma are synovial sarcoma, malignant fibrous histiocytoma, renal angiosarcoma and sarcomatoid variant of RCC [2]. On microscopic examination, hemagiopericytomas show stag horn pattern of vascularity with aggregation of spindle shaped cells around them and minimal collagenisation. There is monotonous cellular proliferation with electron microscopy being helpful in clinching diagnosis. Immunohistochemistry staining is rather important to rule out other solitary fibrous tumours of the kidney. The neoplastic progenitor cells stains usually for vimentin and in a smaller percentage of cases with CD34. Both microscopy and CD 34 studies help in diagnosis of this rare entity [3]. Histological features associated with malignant behaviour are increased cellularity mitotic activity with more than four per ten high power fields, areas of necrosis and haemorrhage [5].
Surgical excision is the treatment of choice for these tumours. Metastasis which is normally by haematogenous route is seen in approximately 15% of cases in various anatomic sites with lung being the most common site of metastasis. The factors according to Enzinger FM and Smith BH which influences survival are related to size, age at diagnosis and histological pattern [5]. Mitoses >4/HPF confers poor prognosis on the patient (10 year survival 77% vs. 29%). Tumour size less than 6.5 cm (ten year survival rate of 95% vs. 63%) and absence necrosis on histology (81% vs 29%) were associated with longer survival. Patients with age less than 40 years had a better chance of survival than those above 40 years [5].
Lymph node dissection, radiotherapy and chemotherapy are not usually recommended with the latter advocated in adjuvant setting. Role of radio and chemotherapy in adjuvant setting is also uncertain with Friedman and Egan stating their experience in which they followed seven patients for 11 years [6]. All patients received radiotherapy after surgery, but only one patient was alive at the end of 11 years (mean age of survival 32 months). Hence, it can be concluded that complete surgical excision might be the only the only factor which limit the occurrence of metastasis [4]. Though, the most common presentation of hemangiopericytoma is mass and pain [6]; however the current patient presented with haematuria and renal imaging was ambiguous with regards to RCC. Only with the help of pathology and immunocytochemistry was the diagnosis made possible. The patent was followed up for a period of 28 months, without any new lesions on CT imaging or haematuria. He continues to be on a single daily dose antihypertensive.
Conclusion
Hemangiopericytoma is rare tumour with difficult preoperative diagnosis due to variable imaging and clinical presentation. Radical nephrectomy is the only preferred treatment with variable outcomes. There have not been favourable responses to either chemotherapy or radiotherapy.
[1]. Filho JE, Bahia LA, Esteves PE, Maron PE, Vedovato BC, Fernandes RD, Renal haemangiopericytoma: case report and literature reviewEinstein (Sao Paulo) 2015 13(2):269-72.10.1590/S1679-45082015RC279325946050 [Google Scholar] [CrossRef] [PubMed]
[2]. Brescia A, Pinto F, Gardi M, Maria Vecchio F, Bassi PF, Renal haemangiopericytoma: case report and review of the literatureUrology 2008 71(4):755.e9-12.10.1016/j.urology.2007.10.06618387402 [Google Scholar] [CrossRef] [PubMed]
[3]. Argyropoulos A, Liakatas I, Lykourinas M, Renal haemangiopericytoma: the characteristics of a rare tumourBJU Int 2005 95(7):943-47.10.1111/j.1464-410X.2005.05443.x15839909 [Google Scholar] [CrossRef] [PubMed]
[4]. Chaudhary A, Seenu V, Sedain G, Ray R, Sharma S, Agarwal S, Haemangiopericytoma of renal pelvis-an unusual tumour in an adolescentUrology 2007 70(4):811.e13-14.10.1016/j.urology.2007.07.03417991570 [Google Scholar] [CrossRef] [PubMed]
[5]. Enzinger FM, Smith BH, Haemangiopericytoma-an analysis of 106 casesHum Pathol 1976 7(1):61-82.10.1016/S0046-8177(76)80006-8 [Google Scholar] [CrossRef]
[6]. Bilici A, Ustaalioglu BB, Seker M, Salman T, Igdem AA, Celik E, Metastatic renal haemangiopericytoma a case reportArch Oncol 2009 17(1-2):32-35.10.2298/AOO0902032B [Google Scholar] [CrossRef]