Trigeminal neuralgia usually presents with significant hemifacial pain or muscle twitching. Though, not a life threatening condition, the morbidity could be quite debilitating, affecting the normal life and work routine. With advances in MR techniques and resolutions, it is possible to identify causes of trigeminal neuralgia more definitively in most cases. Though, the most frequent aetiology is a neurovascular compression, MRI with dedicated cranial nerve sequences has demonstrated a few rarer causes of this condition in this study [1].
Neurovascular compression remains the leading cause of persistent, unilateral trigeminal neuralgia. The clinical significance of the neurovascular compression remained vague in prior studies and hence, confusion existed on which cases required a surgical microvascular decompression [2-4]. The purpose of this study was to try and fill up this lacuna. By grading the neurovascular compression in ascending order of severity in present study, the aim was better segregation of the patients who require surgical microvascular compression.
Furthermore, with this study, other less common causes for this debilitating condition were demonstrated. These aetiologies have not been adequately and comprehensively studied in the setting of trigeminal neuralgia. A potentially reversible aetiology of benign intracranial hypertension causing trigeminal neuralgia forms part of this study.
This study aims in showing that all trigeminal neuralgias are not secondary to neurovascular compression; and other unusual causes need to be looked for, demonstrated and assessed in present MRI examination. This study also aims to prognosticate the severity of the neurovascular compression.
Materials and Methods
This was a cross sectional observational study conducted at the Saveetha Medical College and Hospital, Chennai, India, after obtaining permission from the Institutional Ethics Committee of the Saveetha University. The study period was two years from September 2015 to August 2017. We prospectively studied 70 adult patients who were referred for an MRI brain examination with a clinical diagnosis of unilateral trigeminal neuralgia. This diagnosis was done by neurologists; patients presented with progressively increasing unilateral facial pain, which begun as short mild attacks due to mild stimulation (like brushing/shaving/face washing etc.,) and then progressed in frequency and severity to searing pain. Both male (28) and female (42) patients were included in the study. The age group of the patients was from 34-72 years.
Patients with known dental issues which could cause the pain, and patients with intracranial tumours were excluded from the study. Patients presenting with bilateral trigeminal neuralgia were also excluded from this study.
An informed written consent was obtained from all the patients before performing the study. The MRI was done using a 1.5 Tesla, Philips multiva system. The MRI brain was done using a DWI, T2 axial, 3D Fluid Attenuation Inversion Recovery (FLAIR) and 3D T1 Fast Field Echo (FFE) sequences.
In addition, a high resolution 3D T2 Driven Equilibrium (DRIVE) or 3D balanced Fast Field Echo (bFFE) cranial nerve sequences were performed with the following parameters TR-6.2 ms, TE-3.1 ms, voxel size-0.6, FOV-18×18 cm, matrix-512, flip angle-60°. The MR angiogram was done using a 3D Time-Of-Flight (TOF) technique in selected cases.
Intravenous contrast media (Gadolinium compound-Gadobutrol) was administered only for two patients with parenchymal changes in the pons (Trigeminal pontine sign) for a contrast enhanced MRI. The other 68 patients did not undergo contrast enhanced MRI.
With these techniques, we were able to study the trigeminal nerves in its root entry zone, cisternal segment (prepontine cistern) and the Meckel’s cave segments.
Results
We categorised the patients according to the findings on MRI into patients with neurovascular compression, with benign intracranial hypertension, with trigeminal pontine sign and the last category was a small group of patients with no specific findings on the MRI [Table/Fig-1].
MRI findings in 70 patients.
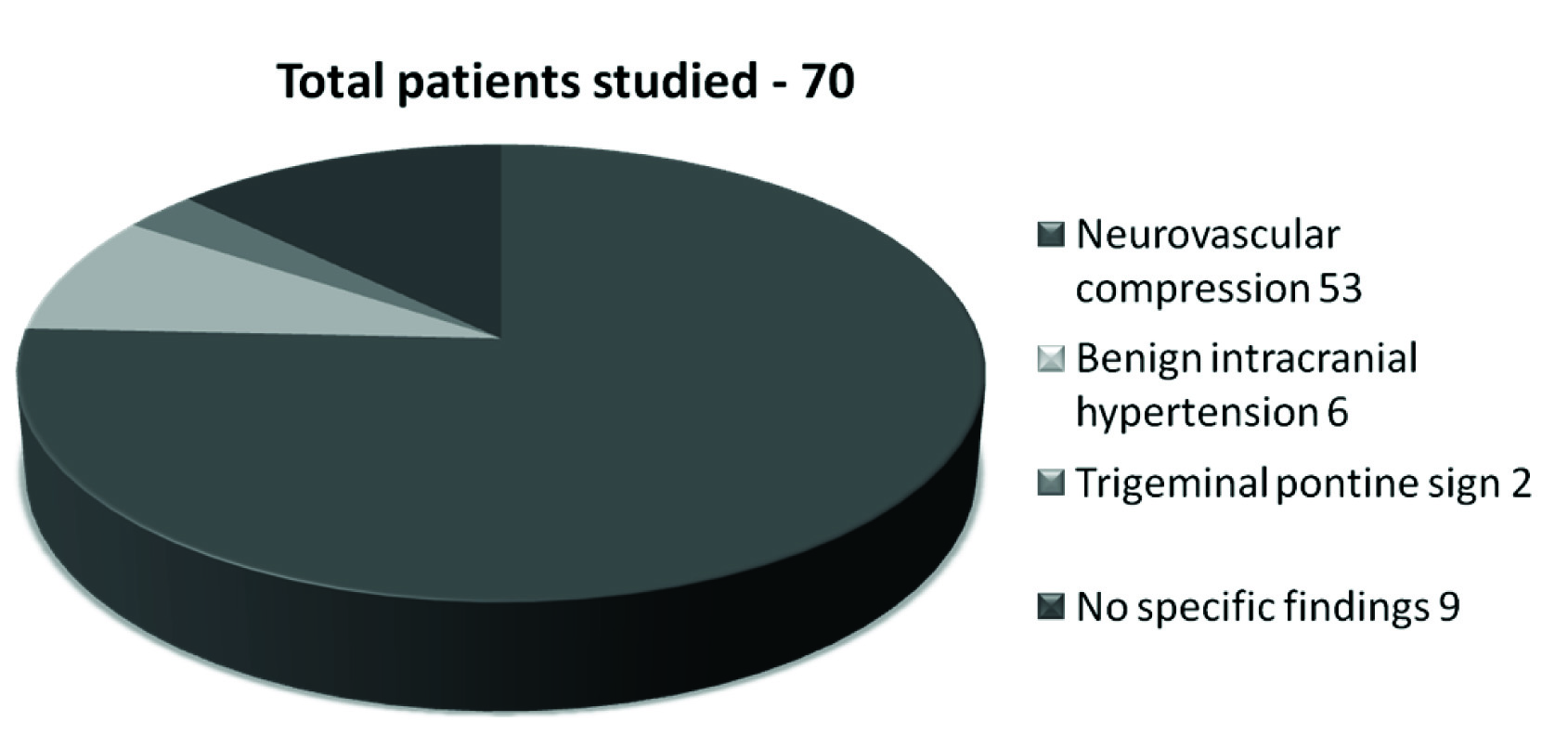
Neurovascular Compression
Out of the 70 patients, we found that the majority (53) had a neurovascular compression in some form.
To classify the neurovascular compression in a more specific manner to aid prognosis and management, a grading system was proposed by the first author; this was named as “MR severity grading of neurovascular compression of the trigeminal nerve”. This grading was based on the severity of the neurovascular compression as demonstrated by the high resolution MRI cranial nerve sequence (3D bFFE and 3D T2 DRIVE). This was to grade the findings in the ascending order of severity [Table/Fig-2].
MR severity grading of neurovascular compression of the trigeminal nerve.
| Grade | Finding |
|---|
| Grade 0 | A vascular loop is seen in the prepontine cistern, but not abutting the nerve |
| Grade 1 | Vascular loop abutting the nerve (root entry zone or the cisternal segment) without nerve displacement or atrophy |
| Grade 2 | Vascular loop causing mild indentation of the nerve |
| Grade 3 | Vascular loop significantly displacing the nerve, without nerve atrophy |
| Grade 4 | Vascular loop significantly displacing/distorting the nerve with signs of nerve atrophy |
The 53 patients with MR features of neurovascular compression were graded from Grade 0 to 4 [Table/Fig-3]:
Categorisation of the 53 patients with MR features of neurovascular compression.
| Out of 53 patients with neurovascular compression |
|---|
| Grade 0 | 0 |
| Grade 1 | 27 |
| Grade 2 | 8 |
| Grade 3 | 11 |
| Grade 4 | 7 |
None of them were in Grade 0.
Patients with Grade 1 type of presentation were 27 out of the 53 patients [Table/Fig-4a,b].
a) Axial bFFE in a patient with right trigeminal neuralgia, showing a vascular loop (orange arrow) abutting the root entry zone of the right trigeminal nerve (blue arrow); b) Sagittal bFFE showing mild abutment of the right trigeminal nerve (blue arrow) by a vascular loop (orange arrow).
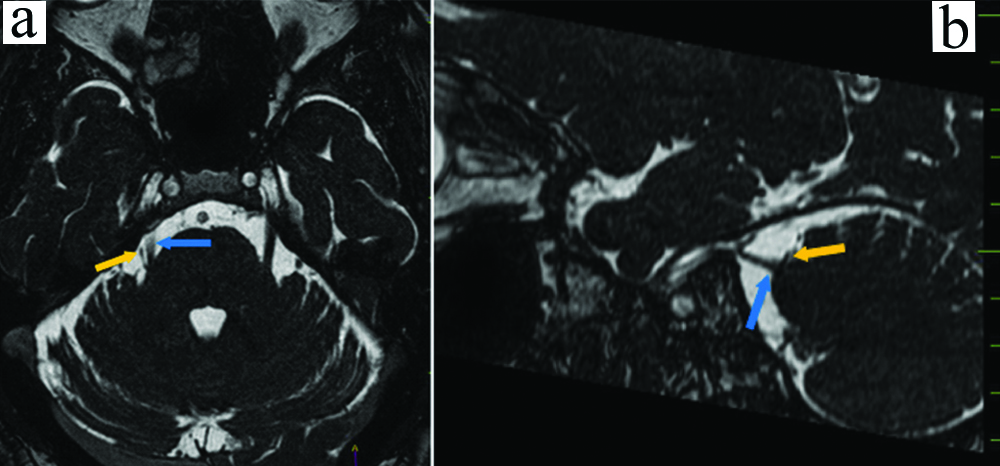
Patients with Grade 2 type of presentation were eight [Table/Fig-5a,b].
a) Axial bFFE showing indentation of the root entry zone of the right trigeminal nerve (blue arrow) by a small vascular loop (orange arrow); b) Sagittal bFFE showing indentation of the root entry zone of the right trigeminal nerve (blue arrow) by a small vascular loop (orange arrow).
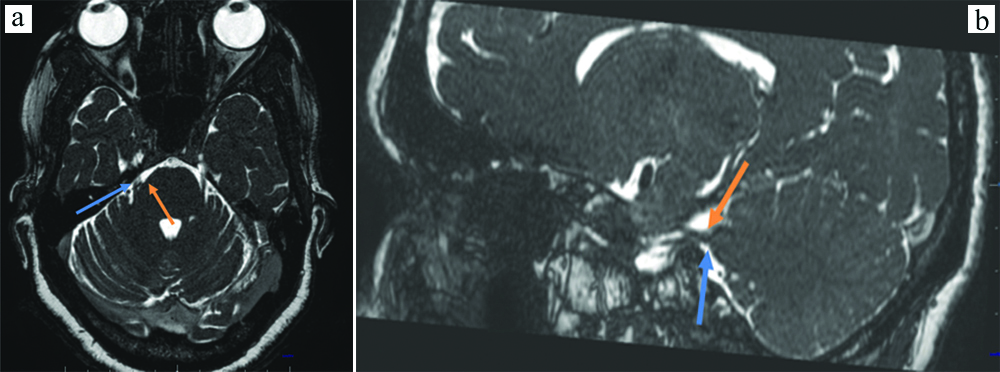
Patients with Grade 3 type of presentation were 11 [Table/Fig-6].
Sagittal bFFE showing indentation and displacement of the root entry zone of the right trigeminal nerve (blue arrow) by a small vascular loop (orange arrow).
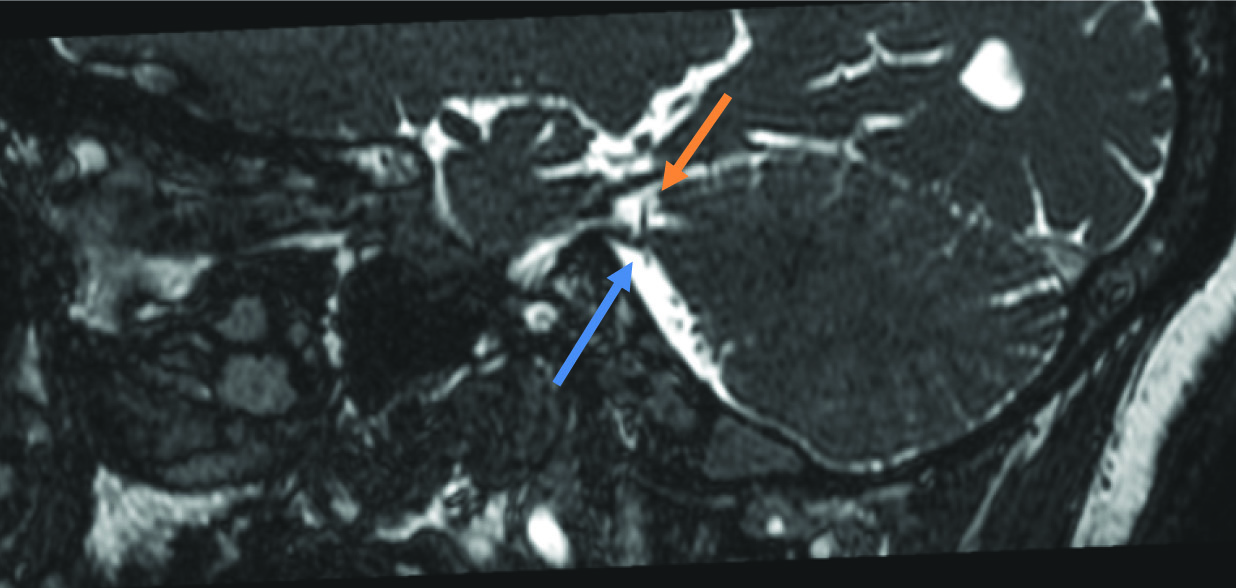
Patients with the most severe Grade 4 presentation were only 7 [Table/Fig-7].
Axial bFFE showing a significant indentation and relative thinning of the root entry zone of the right trigeminal nerve (blue arrow) by a vascular loop (orange arrow).
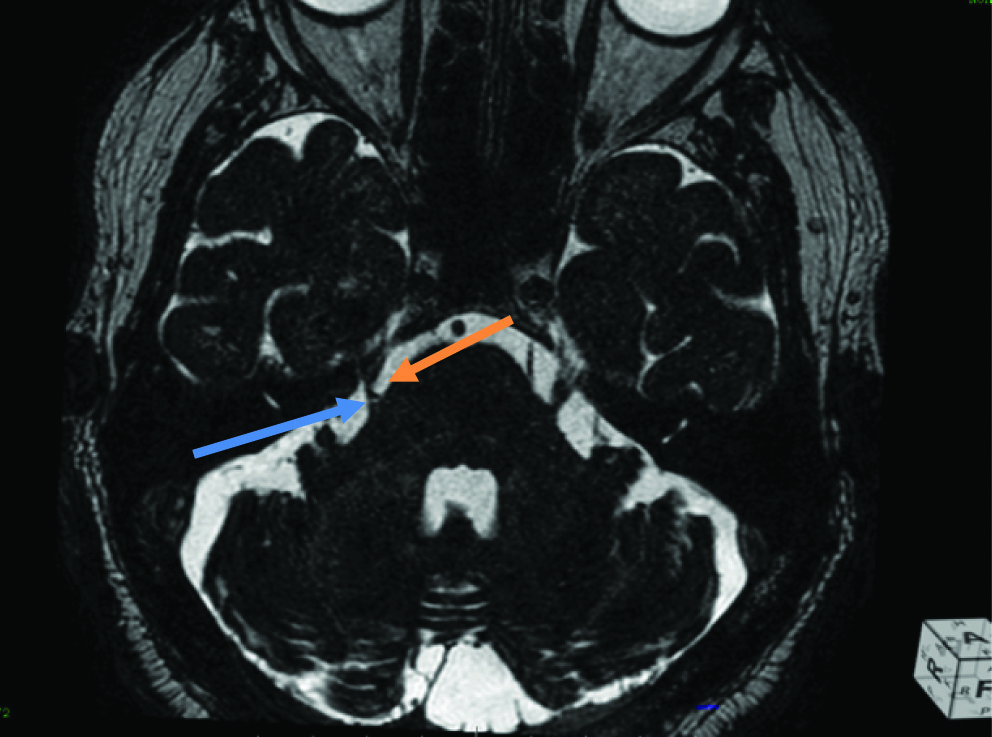
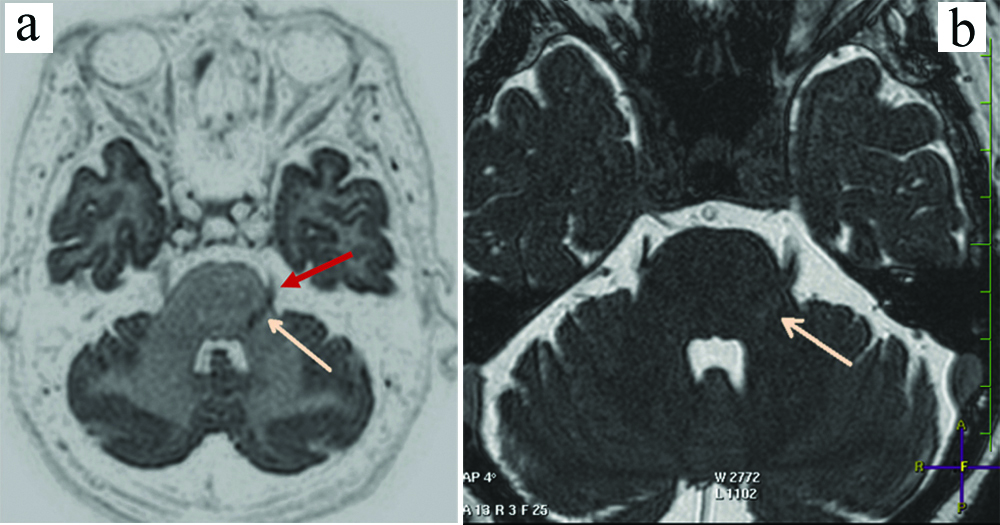
In almost all cases, the indenting vascular loop was a tortuous SCA. There was a small group of nine patients out of these 53 patients who had full blown vertebrobasilar dolichoectasia, where there was significant compression of the root entry zone as well as the proximal cisternal segment by a dolichoectatic basilar artery [Table/Fig-8a,b].
a) Axial bFFE showing a dolichoectatic basilar artery (blue arrow) significantly indenting, compressing and displacing the root entry zone and proximal cisternal segment of the left trigeminal nerve (orange arrow); b) Sagittal bFFE showing a dolichoectatic basilar artery (blue arrow) significantly indenting, compressing and displacing the root entry zone and proximal cisternal segment of the left trigeminal nerve (orange arrow).
Benign Intracranial Hypertension
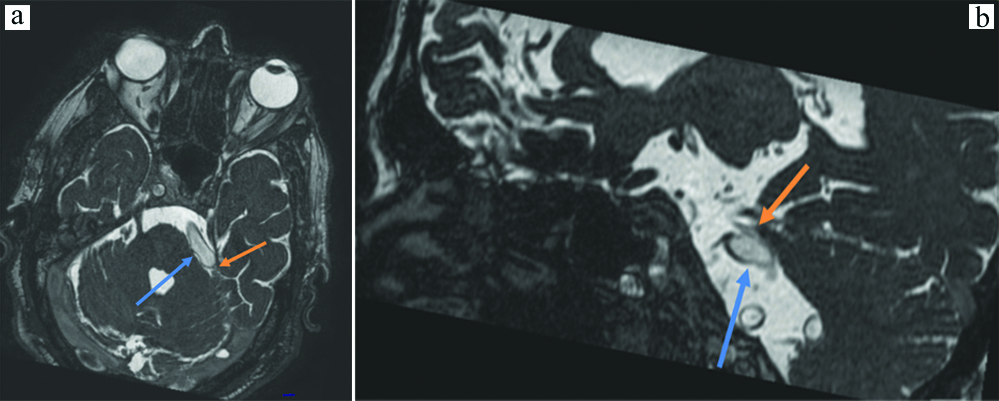
Out of the 70 patients who were studied, six were found to have MR features of benign (idiopathic) intracranial hypertension. All six patients were females, aged between 34-47 years. They had empty sella, with mild prominence of bilateral perioptic Cerebrospinal Fluid (CSF) spaces, optic nerve tortuosities and variable prominence of the bilateral Meckel’s caves [Table/Fig-9a-c]. Other than these findings the MRI brain of these patients did not reveal any significant abnormality.
a) Sagittal bFFE image showing an empty sella (blue arrows); b) Axial bFFE image showing bilaterally prominent Meckel’s caves (blue arrows); c) Axial bFFE showing mildly prominent perioptic CSF spaces.
Trigeminal Pontine Sign
Two patients had an unusual finding of a linear T2 and FLAIR hyperintense signal along the pons, extending from the dorsal pons to the ventral paramedian root entry zone region. There was no diffusion restriction/contrast enhancement of this hyperintensity.
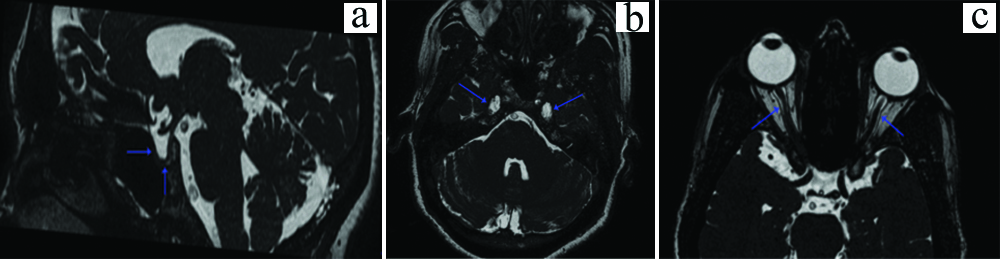
Both the patients were females, one 64-year-old female patient with a right sided trigeminal pontine sign [Table/Fig-10a,b] and a 55-year-old female patient having a left trigeminal pontine sign [Table/Fig-11a,b].
a) Axial T2 image at the level of the pons, showing a linear hyperintensity along the trigeminal tract in the right pons (blue arrow); b) Axial FLAIR image at the level of pons showing the linear hyperintensity (blue arrow) as well as the trigeminal nerve (red arrow).
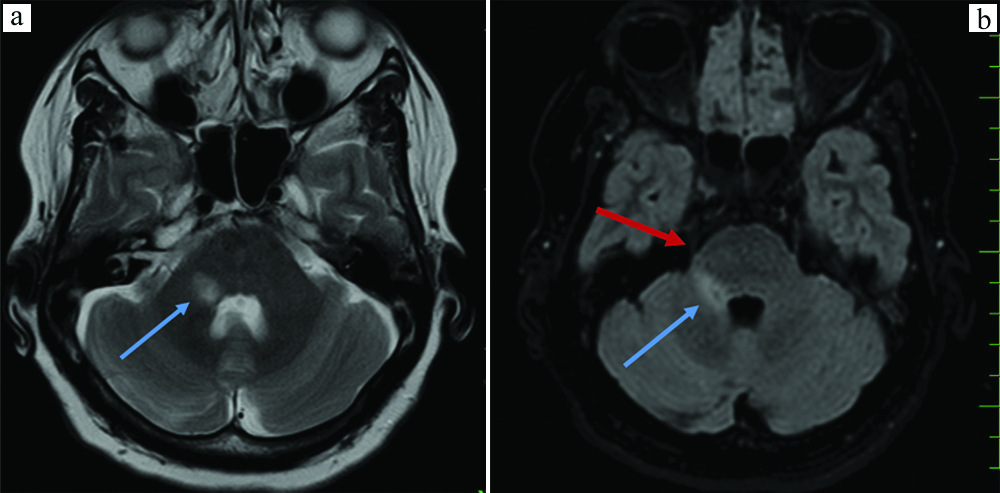
a) Invert FLAIR image showing a left sided trigeminal pontine sign (pink arrow) extending upto the root entry zone of left trigmeninal nerve (red arrow); b) Axial DRIVE image showing a left trigeminal pontine sign (pink arrow).
No Specific Findings
Out of our 70 patients of trigeminal neuralgia, nine patients did not have any specific positive MRI findings. A TOF MR angiogram was also performed, which did not reveal any pertinent abnormality.
Discussion
Based on present results, this discussion is better done under three categories:
Category 1: Neurovascular Compression
Neurovascular compression has been found to be the commonest aetiology of unilateral trigeminal neuralgia [1-5]. However, not all patients with vascular compression of the trigeminal nerve present with neuralgia symptoms. Studies have shown that the proximal (root entry zone and proximal cisternal segment) are the centrally myelinated fibers, which can undergo focal gliosis [6-8]. The distal cisternal segment (peripherally myelinated fibers) compression has been found to be relatively less symptomatic [8]. The transition zone from centrally myelinated to peripherally myelinated fibers also has been found to be vulnerable to compression [7,8]. Out of these 53 patients who had trigeminal neuralgia, 51 had vascular loops close to the centrally myelinated portion of the nerve.
Since, not all cases of neurovascular compression require surgical microvascular decompression, to make present findings of vascular indentation/compression more clinically relevant, we decided to grade present findings in ascending order of the severity [Table/Fig-2] [2].
This grading was done to classify the neurovascular compression, so as to aid the decision on management, whether the patient essentially requires microvascular decompression [4,5]. To best of our knowledge, this is the first such study which graded the neurovascular compression in this manner.
Majority of the patients only had a grade 1 type of indentation. There were seven patients who had a severe compression, with the nerve appearing focally thinned out suggesting gliosis [9]. Interestingly all these seven patients had a proximal nerve compression, which indicated compression of the centrally myelinated nerve fibers. All seven patients had a very intractable trigeminal neuralgia clinically. The other interesting finding was that none of the 53 patients had a finding fitting it into Grade 0, possibly reflecting that if the patient is clinically symptomatic, there had to be some grade of neurovascular contact [10,11].
The patients who had a grade 3 and grade 4 neurovascular compressions would possibly require a surgical microvascular compression. Patients who had Grade 1 neurovascular compression would probably respond to symptomatic medical management. Patients who had a Grade 2 neurovascular compression could be given a trial of medical management, and if they do not respond, surgical microvascular compression would be the next step.
In a study by Sekula RF et al., on safety of microvascular decompression for trigeminal neuralgia in the elderly, concluded that though, microvascular decompression has been regarded as a highly effective and durable treatment for trigeminal neuralgia, there is a relationship between advanced age and the risks of in-hospital death as well [2]. However, severity of compression as demonstrated by imaging was not considered as a criterion before deciding on surgical management. Barker FG et al., in their study found that patients with more severe vascular compression of the trigeminal root had more successful relief of symptoms after microvascular decompression [4]. However, they did not record the severity of compression at the time of operation, and therefore could not make a definitive statement.
The presence of a MR grading system for the severity of vascular compression could therefore definitively help in selecting patients for surgical management and help in establishing a systematic management protocol.
A few (9) patients had a florid vertebrobasilar dolichoectasias where the nerve indentation was by the tortuous basilar artery rather than the superior cerebellar artery. In all other cases, the offending vascular loop was a tortuous superior cerebellar artery. This finding is consistent with prior studies. In this study by Lorenzoni J et al., found that the SCA was involved in 71 cases (76%) [7].
Category 2: Benign Intracranial Hypertension
There were six out of our 70 patients which had features of benign intracranial hypertension, with empty or partially empty sella, prominence of perioptic CSF spaces, optic nerve tortuosities and prominent Meckel’s cave. The atypical presentation of some cases of benign intracranial hypertension presenting with trigeminal neuralgia is a relatively new finding [12]. The aetiology is not very clear and the hypothesis suggested includes compression of the nerves by the increased intracranial pressure, traction of the nerves due to pressure in the posterior fossa and radiculopathy [12-14]. Out of these, the possible mechanical compression of the nerve due to increased intracranial pressure has been suggested to be the most plausible [12,13,15,16]. All six patients were female patients with the youngest being 34 and the oldest 47. And all of them presented with unilateral symptoms. After the MRI diagnosis, the clinical examination of these six patients, revealed findings of papilloedema, and hence were taken up for appropriate management. All six patients had a gratifying, partial to complete resolution of trigeminal neuralgia following lumbar puncture.
Category 3; Trigeminal Pontine Sign
The most interesting and unusual finding were present in two patients, who presented with a “Trigeminal pontine sign” [17,18]. Previous studies done for trigeminal neuralgia have shown a very small group of people presenting with linear brainstem lesions along the trigeminal nerve course. When evaluated, these patients either had Multiple Sclerosis (MS) or a herpetic infection [17]. Both our patients were middle aged female patients with no clinical or imaging findings to suggest a demyelinating pathology. However, one patient had a history of childhood varicella infection. The other patient was not very confident on whether she had had it in childhood. Both of them had an obliquely linear T2 and FLAIR hyperintense signal along the descending trigeminal tract in the pons. Earlier studies have suggested the hypothesis of an inward migration of the varicella zoster virus, from the Gasserian ganglion to the pontine fibers, progressing on to degenerative demyelination and gliosis [17,19]. Very few such cases have been reported in literature and we had two such patients in present study [17,18].
We could not demonstrate a cause for 9 out of the 70 patients studied. MRI brain as well as the MR angiogram revealed no definite abnormality.
Limitation
The limitation of the study was that this was done in a 1.5 Tesla MRI machine. With the advent of higher strength MRIs like 3 Tesla and even 7 Tesla, it could be possible to get an even better resolution image of the cranial nerves in a higher strength MRI. This could help in demonstration of further aetiologies, or better demonstration of the already described aetiologies. Hopefully such studies would be done in the future.
Conclusion
The clinically significant hallmark of this study is the grading of the neurovascular compression to specifically segregate the patients who require surgical microvascular compression and achieve a prognostic significance. Other authors are welcomed to use the current grading system and validate it under different settings and population.
Identifying reversible aetiologies such as benign intracranial hypertension helps the clinician in providing complete alleviation of patient symptoms. In most cases of trigeminal neuralgia, both common and uncommon causes can be demonstrated on a tailored MRI and consequently aid the clinician for appropriate management.