Propofol, which was approved by the Food and Drug Administration (FDA) in 1993 for use as a sedative for mechanically ventilated patients in the Intensive Care Unit (ICU), is an intravenous phospholipid emulsion that has anaesthetic, sedative, and hypnotic properties [9]. Propofol has a rapid onset of action and short duration of sedation once discontinued; therefore, pharmacokinetically, it is a favourable choice for use in cardiac surgery [10].
Bispectral analysis of the Electroencephalography (EEG) is a signal processing technique that has been proposed as a pharmacodynamic measure of anaesthetic effects on the central nervous system [11]. Recently, the BIS has been shown to measure the hypnotic component of the anaesthetic state [12]. There are very few studies comparing dexmedetomidine with a known anaesthetic adjuvant such as propofol in open heart surgeries. Hence we undertook this study to evaluate the effect of dexmedetomidine on intraoperative requirements of isoflurane and opioids for maintenance of anaesthesia during On-pump cardiac surgery and compare the anaesthesia sparing effect with that of propofol.
Materials and Methods
This was a randomised, double blind observational study conducted over a period of two years (June 2014 to June 2016). The study was approved by the Institutional Ethical Committee. All the patients gave informed consent to participate in this study. The study included 60 patients aged 15 years and above with American Society of Anesthesiologists (ASA) status I-III, planned for valve replacement or repair surgeries. The exclusion criteria were severe cardiac disease (New York Heart Association class IV (left ventricular ejection fraction less than 40%), coexisting systemic disease (severe renal or hepatic dysfunction-creatinine >2 mg/dL and serum glutamate phosphoribosyl transferase >150 units), long term use of sedatives, analgesics and tricyclic antidepressants, psychiatric illness, alcohol abuse, allergy to propofol or dexmedetomidine. Also, patients who required deep hypothermic circulatory arrest were excluded.
A computer generated randomisation table was used to assign the patients to the dexmedetomidine group (n=30) or the propofol group (n=30). An anaesthesiologist not associated with the study prepared injectable infusions containing either dexmedetomidine or propofol. The investigator was not involved with conduct of anaesthesia and was present for data collection purpose only. All the patients were premedicated with oral diazepam 5 mg 12 hours prior to surgery. A large bore cannula (18G) was inserted for fluid and drug administration. Routine monitoring consisted of non invasive blood pressure, electrocardiography, peripheral oxygen saturation (SpO2) monitoring, oesophageal temperature, neuromuscular monitoring and urine output. Four BIS disposable sensors (Aspect Medical Systems, Inc Norwood, MA 02062 USA) were applied to forehead one over each outer malar bone, the third at centre of forehead and fourth on one side of third electrode. BIS displayed on the monitor Mindray (China) WATO EX-65 was recorded. Induction of anaesthesia was achieved with thiopentone 3-6 mg/kg and morphine 0.1-0.2 mg/kg. Vecuronium 0.1 to 0.2 mg/kg was given to facilitate tracheal intubation. Each patient of dexmedetomidine group received an initial bolus dose of 1 μg/kg over 10 minutes followed by infusion at rate of 0.2 to 0.6 μg/kg/hour. Each patient of propofol group received infusion of propofol at rate of 0.25-1 mg/kg/hour. Intraoperatively, blinding to propofol infusion was achieved by shielding medications, infusion tubings and intravenous site from the view of everyone. Central venous catheter and arterial cannula were inserted for measurement of central venous and invasive blood pressure. Anaesthesia was maintained with isoflurane Minimum Alveolar Concentration (MAC) 1.2 in 100% oxygen. Muscle relaxation was supplemented with vecuronium bromide (0.01 to 0.02 mg/kg). Target BIS was kept between 40-60. The study drug infusions were started with the lowest allowable dose as per study protocol and titrated to target BIS range. Any deviation above this range was addressed by deepening the depth of anaesthesia by increasing dose of study drugs, MAC of isoflurane and administration of additional doses of morphine (0.05 mg/kg). Similarly doses of study drugs and MAC of isoflurane were decreased for BIS <40. Heart rate and mean arterial pressure were kept within 25% of baseline. Tachycardia and hypertension were treated with beta blockers and vasodilators. Maquet HL20 Cardiopulmonary AG Kehler Strabe 81, 76437 Rastat (Germany) and membrane oxygenator were used for Cardiopulmonary Bypass (CPB). After aortic root cannulation, other necessary cannulae were placed and moderate hypothermic CPB (28-32°C) was initiated. Lungs were not ventilated during full cardiopulmonary bypass flows. Mean systemic perfusion pressure was maintained between 50-80 mmHg during CPB and any hypertension/hypotension was treated with nitroglycerine or phenylephrine boluses. Myocardial protection was achieved with potassium rich blood cardioplegia and topical application of ice slush and cold saline. Patient’s temperature was maintained within desired range using heat exchanger. The fresh gas flow using alpha stat strategy was adjusted to maintain the normal acid-base balance. Rewarming was performed for 25 minutes at the rate of 1°C per 3-5 minutes to 37°C. Isoflurane was used before and after CPB as at our institute it was neither usual nor feasible to add isoflurane to the CPB circuit. Infusions of propofol or dexmedetomidine were continued during CPB. After skin closure drug infusions and isoflurane were stopped patients were shifted to cardiothoracic ICU for elective mechanical ventilation. Patients were weaned from pressure regulated volume control to pressure support mode of ventilation as soon as they were conscious, had adequate breathing effort and were haemodynamically stable. Patients were extubated when standard criteria for weaning and extubation were met.
The total dose of morphine required in the study groups was recorded. MAC was recorded at the following time intervals during surgery-post intubation, at sternotomy, before initiation of CPB, post weaning from CPB, at skin closure and at end of surgery.
Statistical Analysis
All the continuous variables of this study have been shown as Mean±Standard Deviation (SD) and categorical variables have been shown in terms of frequency and percentages. Continuous data were analysed with Student’s t-test or Mann-Whitney U test. Analysis of variance was used to analyse the variables measured on repeated time points. A probability value (p-value) less than 0.05 was considered significant. SPSS version 20.0 (SPSS Inc., Chicago, IL) was used to analyse the data.
Results
Sixty patients were evaluated, 30 patients in each group and no patient was excluded at any stage of the study. The demographic data regarding age, gender distribution in study patients, weight, ASA physical status, comorbidities (hypertension and diabetes mellitus), left ventricular ejection fraction and CPB time was similar in both the study groups [Table/Fig-1].
| Parameters | Dexmedetomidine group | Propofol group | p-value |
|---|
| Age (years) | 33.6±11.816 | 35.56±9.54 | 0.48 |
| Gender distribution (M/F) | 12 (60%)/18 (45%) | 8 (40%)/22 (55%) | 0.99 |
| Weight (kg) | 55.5±9.83 | 53.63±8.31 | 0.43 |
| ASA status (I,II) | 13 (50%)/17 (50%) | 13 (50%)/17 (50%) | 1.00 |
| Hypertension | 8 (50%) | 8 (50%) | 1.00 |
| Diabetes mellitus | 0 (0%) | 1 (100%) | 1.00 |
| LVEF (%) | 65.96±7.36 | 64.13±4.09 | 0.23 |
| CPB time (in minutes) | 105.43±48.69 | 118.93±52.49 | 0.30 |
Data are expressed as mean±SD and numbers as percentage (%)
M/F: Male/Female, ASA: American Society of Anesthesiologists, CPB-Cardiopulmonary Bypass time
Morphine requirement to maintain adequate depth of anaesthesia and to prevent haemodynamic response to painful stimuli (laryngoscopy and sternotomy) during the pre-bypass period was significantly lower in dexmedetomidine group (p<0.05). During CPB and after weaning from CPB, patients in dexmedetomidine group required significantly less dosage of morphine to maintain BIS scores within the desired anaesthetic range of 40-60 [Table/Fig-2].
Comparison of dose of morphine (mg) used in study groups.
| Time interval | Dexmedetomidine group | Propofol group | p-value |
|---|
| Before cardiopulmonary bypass | 5.05±1.27 | 5.95±0.62 | 0.001 |
| During CPB | 5.1±1.50 | 6.4±1.62 | 0.002 |
| After weaning from CPB | 2.95±1.39 | 3.7±1.29 | 0.034 |
Dose of morphine required in dexmedetomidine group was significantly lower compared to propofol group at all stages of the surgery (p<0.05). Data are expressed as mean±SD. CPB-Cardiopulmonary Bypass
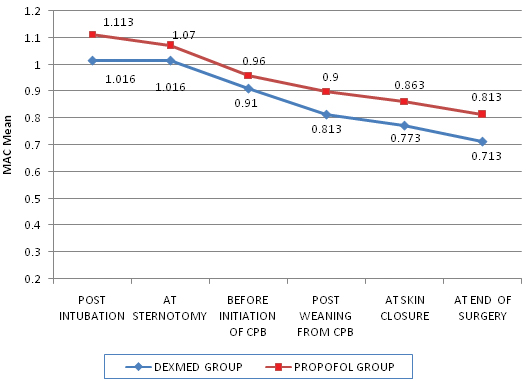
In our study, during the intraoperative period isoflurane concentrations were adjusted to target BIS of 40-60 in addition to altering study drug infusion rates. In dexmedetomidine group, MAC post intubation was 1.016±0.11 compared to 1.113±0.08 in propofol group, the difference being statistically significant (p<0.05). At all the stages of surgery i.e., sternotomy, before initiation of CPB, post weaning from CPB, at skin closure and at end of surgery MAC required in dexmedetomidine group continued to be significantly lower (p-value <0.001) than that of propofol group [Table/Fig-3].
Line diagram showing MAC of isoflurane in the study groups.
MAC requirement to keep bispectral scores within 40-60 was significantly lower in dexmedetomidine group (p<0.05). MAC-Minimum Alveolar Concentration, CPB-Cardiopulmonary Bypass
BIS scores in both the groups at the predefined intervals were within target range of 40-60. Pre and post CPB BIS scores [Table/Fig-4] were statistically lower in dexmedetomidine group than propofol group and the difference was significant (p<0.05).
Bispectral index trends between the study groups.
| Time interval | Dexmedetomidine group | Propofol group | p-value |
|---|
| Post intubation | 42.0±2.8 | 48.5±6.4 | <0.001 |
| Sternotomy | 49.1±3.9 | 52.7±3.9 | <0.001 |
| Before initiation of CPB | 48.4±5.0 | 51.4±4.3 | 0.014 |
| Post weaning from CPB | 47.6±4.7 | 50.2±4.3 | 0.027 |
| At skin closure | 45.7±4.3 | 49.6±5.7 | 0.004 |
| At end of surgery | 44.5±5.5 | 48.2±5.3 | 0.011 |
Bispectral index scores remained between target range of 40-60 and were significantly lower in dexmedetomidine group.
BIS:Bispectral index, MAC: Minimum Alveolar Concentration, CPB: Cardiopulmonary Bypass
Discussion
The volume of patients requiring cardiac surgery has increased substantially, with an unparalleled growth in hospital resources. This imbalance has made facilitated recovery also known as fast-track recovery, an increasingly popular option after cardiac surgery. Fast-track recovery incorporates modifications in intraoperative anaesthetic management (e.g., balanced anaesthesia as opposed to high dose narcotics) along with advances in myocardial protection (e.g., decreased ischaemia) and perfusion techniques (e.g., temperatures above 32°F) with the goal of facilitating early extubation. Excessive opioid use can lead to a decrease in mental status and respiratory depression making early extubation difficult to perform. The opioid sparing effects of dexmedetomidine coupled with the arousable and interactive state that is elicited make dexmedetomidine an attractive option for patients undergoing major cardiothoracic surgery [13].
Our study showed that consumption of morphine and isoflurane during the intraoperative period, was significantly lower in the dexmedetomidine group than in the propofol group. The results were in accordance to other previously done studies. Khalil MA and Abdel Azeem MS studied 50 patients undergoing Off-pump Coronary Artery Bypass (OPCAB) surgery [14]. The total morphine consumption of patients receiving dexmedetomidine infusion (0.5 μg/kg/hour; dexmedetomidine group), after induction of general anaesthesia, were compared with those receiving placebo (saline group). Fentanyl and morphine consumptions were lower in the dexmedetomidine group than in the control group (p<0.05). They concluded that dexmedetomidine might be an effective adjuvant in reducing total fentanyl and morphine requirements in OPCAB. Herr DL et al., compared dexmedetomidine based to propofol based sedation after Coronary Artery Bypass Graft (CABG) surgery in the intensive ICU [15]. They reported that morphine use was significantly reduced in the dexmedetomidine group. Only 28% of the dexmedetomidine patients required morphine for pain relief during ventilation versus 69% of propofol based patients (p<0.001). Gurbet A et al., studied 50 patients who were randomly assigned to two groups [16]. Group D (n=25) received an intravenous loading dose of dexmedetomidine 1 μg/kg during induction of anaesthesia, followed by a continuous infusion at a rate of 0.5 μg/kg/hour throughout the surgery. Group P (n=25) received a volume-matched bolus and infusion of normal saline. They concluded that continuous intraoperative infusion of dexmedetomidine reduced the narcotic requirements.
Dexmedetomidine has been shown to decrease the MAC of halothane to the extent that it may act as an anaesthetic by itself at high doses in animal studies [17]. The mechanism of the hypnotic action of α2-adrenergic agonists has been attributed to inhibition of adenylate cyclase [18] with consequent changes in transmembrane ion conductance and hyperpolarization of excitable neural cells [19]. In our study MAC of isoflurane at all stages of surgery was significantly lower in dexmedetomidine group (p<0.05). MAC sparing effect of dexmedetomidine has been documented by various studies. Lawrence CJ and De Lange S investigated the effect of a single pre-induction intravenous dose of dexmedetomidine 2 μg/kg on anaesthetic requirements in 50 patients undergoing minor orthopaedic and general surgery procedures [20]. The authors achieved a reduction in isoflurane requirements of 90% in dexmedetomidine treated patients. Keniya VM et al., assessed the efficacy of dexmedetomidine in reducing intraoperative anaesthetic requirement among 60 patients scheduled for elective surgery of more than three hours [21]. Fentanyl requirement during the operation was 100±10 μg in the control group and 60±10 μg in the dexmedetomidine group. The need for isoflurane was decreased by 32% respectively in the dexmedetomidine group as compared to the control group. The authors concluded that dexmedetomidine has significant anaesthetic and opioid sparing effect. Aantaa R et al., concluded that clinically relevant doses of dexmedetomidine induce a dose dependent and significant reduction of isoflurane MAC in patients scheduled for abdominal hysterectomy {with high dose of dexmedetomidine (steady state plasma concentration of 0.6 ng/mL), the MAC of isoflurane was 47% less than that without dexmedetomidine} [22].
Limitation
Limitations of our study include a low sample size, patients with very low ejection fraction and those having poor cardiovascular functional status, who were not included. Such cases are at high risk for developing bradycardia and hypotension during administration of dexmedetomidine. Also intraoperatively only BIS was used to monitor the degree of hypnosis and that can be confounded by many factors. Analgesic requirement in postoperative period was not taken into consideration as the study did not include the postoperative period. It should be noted that further studies with larger sample size are necessary to better define advantages and disadvantages of dexmedetomidine regarding the intraoperative anaesthetic use.
Conclusion
The present study concluded that intraoperatively administered dexmedetomidine during On-pump cardiac surgery has specific analgesic properties and decreases intraoperative anaesthetic requirement. Thus, dexmedetomidine has significant opioid and anaesthetic sparing property.
Data are expressed as mean±SD and numbers as percentage (%)
M/F: Male/Female, ASA: American Society of Anesthesiologists, CPB-Cardiopulmonary Bypass time
Dose of morphine required in dexmedetomidine group was significantly lower compared to propofol group at all stages of the surgery (p<0.05). Data are expressed as mean±SD. CPB-Cardiopulmonary Bypass
Bispectral index scores remained between target range of 40-60 and were significantly lower in dexmedetomidine group.
BIS:Bispectral index, MAC: Minimum Alveolar Concentration, CPB: Cardiopulmonary Bypass