Effectiveness of Tranexamic Acid for Reducing Postpartum Blood Loss in the First Two Hours after Vaginal Delivery: A Randomised Controlled Trial
Anuchat Sujita1, Srisuda Songthamwat2, Metha Songthamwat3
1 Resident, Department of Obstetrics and Gynaecology, Udonthani Hospital, Mueang, Udonthani, Thailand.
2 Head, Department of Obstetrics and Gynaecology, Udonthani Hospital, Mueang, Udonthani, Thailand.
3 Teacher, Department of Obstetrics and Gynaecology, Udonthani Hospital, Mueang, Udonthani, Thailand.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Metha Songthamwat, Teacher, Department of Obstetrics and Gynaecology, Udonthani Hospital, Udonthani-41000, Mueang, Thailand.
E-mail: Udonhome@yahoo.com
Introduction
Postpartum Haemorrhage (PPH) is the leading direct cause of maternal death worldwide, especially in developing countries. Fibrinolysis is an important process in bleeding during the third stage of labour. Tranexamic Acid (TA) is used to reduce the fibrinolysis process which might reduce the blood loss after delivery.
Aim
To study the effectiveness of intravenous TA for reducing postpartum blood loss in the first two hours after vaginal delivery.
Materials and Methods
A prospective double blinded Randomised Controlled Trial (RCT) was performed. The participants were randomly allocated to receive either an intravenous infusion of TA (n=75) or a placebo (n=75) after delivery of the anterior shoulder. A prophylactic intramuscular injection of 10 units of oxytocin was used in both groups. Blood loss was directly measured using a collective bag combined with a gravimetric of gauzes and diapers during the first two hours postpartum. The means of blood loss of both groups were compared. The prevalence of PPH (>500 mL) and severe PPH (>1,000 mL) in both groups were analysed. Statistical analysis were performed using Stata13 (Stata Corp, College Station, TX). A p-value below 0.05 was considered statistically significant.
Results
Seventy two participants in the TA group and 69 participants in the placebo group completed this study. Mean blood loss in the first two hours for the TA group was not significantly different from the placebo group (226.59±114.66 mL versus 234.05±142.41 mL, p=0.73). Adjusted mean difference was 4.61 mL (95% CI: -48.25 to 39.02). The frequency of PPH was one case in the TA group and three cases in the placebo group (one case was severe PPH). Only one woman had a mild side effect (nausea) and no episode of thrombosis occurred in the women who received TA.
Conclusion
In normal delivery, the addition of TA did not reduce the amount of postpartum blood loss in the first two hours compared with prophylaxis oxytocin only.
Postpartum haemorrhage, Prevention, Tranexamic acid
Introduction
The PPH is blood loss after delivery of more than 500 mL by vaginal delivery or more than 1000 mL by cesarean delivery within the first 24 hours [1]. The PPH is the leading direct cause of maternal death worldwide (representing 27.1%) and in South Eastern Asia 29.9% of maternal death are due to PPH [2]. Moreover, PPH can also follow with many complications such as shock, renal failure which increase the hospital stay length and the cost of treatment.
The first one or two hours immediately following delivery is the critical time. It has been defined by some as the “fourth stage” of labour [3, 4]. The PPH as the result of uterine atony is most likely at this period [3]. The World Health Organization (WHO) in 2012 recommended the use of an uterotonic agent after delivery to reduce postpartum blood loss and PPH. An uterotonic agent, preferably 10 units of oxytocin administered intramuscularly immediately after all births, was suggested as the main agent for all postpartum women [5].
The TA is an analogue of lysine that acts as an antifibrinolytic by competitively binding sites on plasminogen molecules and has effectiveness for haemostasis. Therefore, fibrinolysis is inhibited and blood loss is reduced by TA [6]. Usually, TA is used to prevent surgical bleeding in cardiovascular and hepatobiliary tract surgery [7].
The third stage of vaginal delivery has many physiologic processes for reducing postpartum blood loss such as myometrial contractions, increased release of coagulant factors and fibrinolytic activities which are occurring simultaneously [8]. Oxytocin administration enhances the myometrial contraction mechanism while administration of TA might be able to counter the fibrinolytic process and thus facilitate haemostasis. Therefore, TA may help reduce postpartum blood loss in this period.
The treatment role of TA in PPH was proven in a recent large RCT [9,10]. The prophylactic role of TA in PPH was also mentioned in a Cochrane systematic review and a meta analysis study [11]. However, when we looked in detail at this meta analysis, we found that most cases in the study were done in cesarean section studies [12,15]. Few RCTs of prophylaxis TA in vaginal delivery were included in this meta analysis with only a small number of PPH [16,18]. Therefore, we conducted this study aimed at clearly evaluating the effectiveness of intravenous TA for reduction of postpartum blood loss in the first two hours after vaginal delivery.
Materials and Methods
This study was a randomised, double blind, placebo controlled trial which was conducted from January 2016 through February 2017 at the Department of Obstetrics and Gynaecology, Udonthani Hospital, Udonthani, Thailand. The study was conducted after approval by the Ethical Research Committee of Udonthani Hospital No.18/2559. Women in labour who met the inclusion criteria were invited to participate. We explained the study methods and side effects of the drug to the patients then a written informed consent was obtained from all the participants. The inclusion criteria were age more than 18 years, gestational age between 36 and 42 weeks, singleton, a live fetus, cephalic presentation in the active phase of labour (cervical dilate more than 3 cm). Patients with high risk factors for PPH, such as polyhydramnios, fetal macrosomia, grand multiparity (five or more), chorioamnionitis and previous PPH were also included. Exclusion criteria included multiple pregnancy, dead fetus in utero, vacuum extraction, forceps extraction, placenta previa, placenta accreta syndrome, placental abruption, prior cesarean section, or any uterine scar, history of thromboembolic disease, heart disease, liver and renal disorders. The participants were randomly allocated into two groups to receive an intravenous infusion of TA (n=75) or a placebo (n=75). All participants were examined at admission with complete blood count, Blood Urea Nitrogen (BUN) and creatinine. They were then randomised into one of the two study groups using block randomisation by computer generated random number. Intervention drug was labeled and sealed in opaque envelopes. The experimental group’s drug contained 1 gm/10 mL TA diluted with 20 mL of normal saline and the placebo group’s drug contained 30 mL of normal saline. Investigators and patients were blinded to the contents of the intervention drug until the conclusion of the study. We used prophylactic injection of 10 units of oxytocin in both groups then either TA or a placebo was administered intravenously within one minute period at delivery in the anterior shoulder. Standard management of the third stages of labour were performed. The primary outcome measure was the volume of postpartum blood loss during the first two hours after delivery. The other outcomes included the incidence of PPH (more than 500 mL), severe PPH (more than 1,000 mL), need for blood transfusion, need for additional uterotonic drugs (200 μg intravenous methylergometrine and/or 800-1,000 μg misoprostol rectally and intravenous sulprostone) and side effects of TA injection. The mild adverse effects of TA (nausea/vomiting, diarrhea, pyrexia, headache) and the severe adverse effects of TA (deep vein thrombosis, pulmonary embolism, seizure, renal failure) were recorded [19]. Total amount of blood loss (mL) was determined as the sum of: 1) collective bag 2) The gravimetric of gauze (wet weight−dry weight) and 3) Diapers used during the first two hours postpartum (wet weight−dry weight)/1.05 [16,20]. The power calculation was based on the previous study. Twenty five percent reduction of volume of blood loss was used and the sample size calculation was performed by the formula for RCT for continuous data [21]. Mean in a treatment group is 261.50 and SD in a treatment group is 146.80. Mean in a control group is 349.98 and SD, in a control group is 188.85 [16]. The level of significance was considered to be 0.05 and power is 80%. Numbers of participant by calculation were 58 participants per groups. An additional 17 subjects per groups were recruited to study for possible drop out from study.
Statistical Analysis
The study and control group data were compared using an unpaired Student t-test for continuous variable and chi-square and fisher’s exact tests were used for categorical variables. Mean difference with a 95% CI was calculated for magnitude of effect. Statistical analysis were performed using Stata13 (Stata Corp, College Station, TX). A p-value below 0.05 was considered statistically significant.
Results
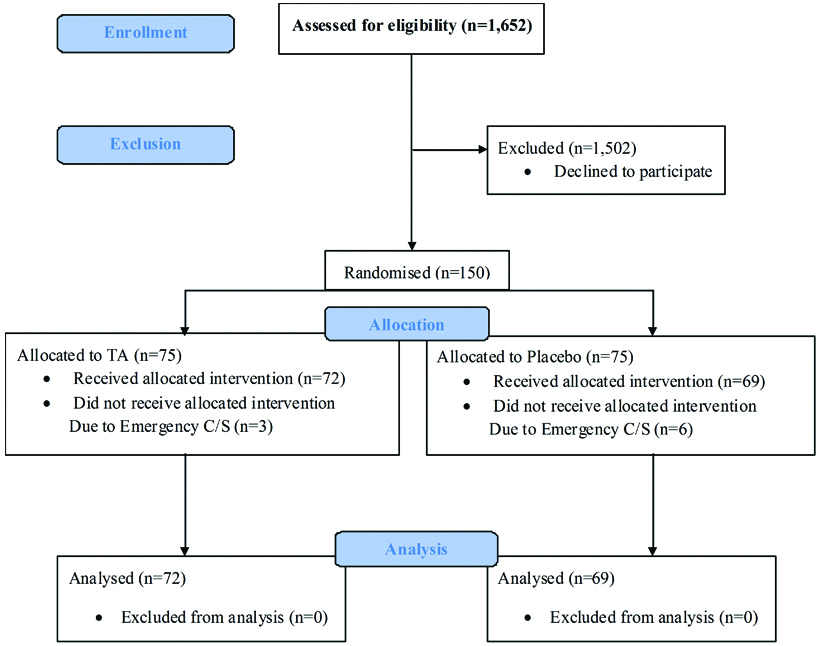
The study included 150 participants allocated in to two groups of 75 participants per group. Seventy two participants in the TA group and 69 participants in the placebo group completed this study. Three participants in the TA group and six participants in the placebo group were excluded from this study due to cesarean delivery from cephalopelvic disproportion [Table/Fig-1]. Both groups were comparable in patient characteristics and there was no significant difference in risk factors for PPH between the two groups except maternal body weight [Table/Fig-2]. The results for primary outcomes and secondary outcomes are presented in [Table/Fig-3]. Mean blood loss in the first two hours for the TA group was not significantly different from the placebo group (226.59±114.66 mL versus 234.05±142.41 mL, p=0.73). Adjusted mean difference was 4.61 mL (95% CI: -48.25 to 39.02).
Flow of participants through the trial.
Comparison of baseline characteristics between two groups.
| Baseline characteristics | TA (n=75) | Placebo (n=75) | p-value |
|---|
| Age (years)* | 24.50±4.94 | 25.52±5.75 | 0.25 |
| Gestational age (weeks)* | 38.70±1.11 | 38.67±1.24 | 0.87 |
| Parity* | 1.85±0.96 | 1.84±1.03 | 0.99 |
| Body weight (kg)* | 63.41±8.81 | 67.45±11.76 | 0.02 |
| Height (cm)* | 158.27±5.44 | 158.76±6.60 | 0.61 |
| Duration of first stage of labour (min)* | 505.44±340.54 | 497.07±278.19 | 0.87 |
| Duration of second stage of labour (min)* | 11.06±6.54 | 12.26±7.97 | 0.33 |
| Duration of third stage of labour (min)* | 6.43±3.47 | 5.98±2.75 | 0.40 |
*values are in mean±SD. p-value was calculated by unpaired student t-test
TA: Tranexamic acid
Comparisons of outcome between two groups.
| Outcome | TA(n=72) | Placebo(n=69) | Mean difference | Adjusted mean difference* | 95% CI | p-value |
|---|
| Total blood loss (mL)† | 226.59±114.66 | 234.05±142.41 | 7.46 | 4.61 | -48.25 to 39.02 | 0.73 |
| Blood loss >500 mL‡ | 1 (1.38%) | 3 (4.34%) | - | - | - | 0.35 |
| Blood loss >1,000 mL‡ | 0 | 1 (1.44%) | - | - | - | 0.35 |
| Blood transfusion‡ | 0 | 1 (1.44%) | - | - | - | 0.30 |
| Use of other uterotonic agent‡ | 2 (2.77%) | 6 (8.69%) | - | - | - | 0.12 |
*Adjusted for body weight
†values are in mean±SD. The p-value was calculated by an unpaired student t-test
‡values are in n (%). The p-value was calculated by chi-square and fisher’s exact tests
CI: Confidence interval, TA: Tranexamic acid
The frequency of PPH was one case in the TA group and three cases in the placebo group (one case was severe PPH). More women in the placebo group (n=6) than in the experimental group (n=2) required additional uterotonic agents but not statistically significant. There was no major complication, (maternal mortality or surgical intervention) for PPH, in either group. Maternal adverse events are summarised in [Table/Fig-4]. Only one woman had a mild side effect (nausea) and no episode of thrombosis occurred in the women who received TA.
Comparisons of side-effects between two groups.
| Side-effects | TA(n=72) | Placebo(n=69) | p-value |
|---|
| Mild adverse effects of TA* | 1 (1.38%) | 0 | 0.32 |
| Severe adverse effects of TA | 0 | 0 | 0 |
*values are in n (%). Using a chi-square and fisher’s exact tests
Total side effect is one case (Nausea)
TA: Tranexamic acid
Discussion
The PPH is the major direct cause of maternal death worldwide, especially in developing countries. The third stage of labour is an important period because placental delivery has a rapid degradation of fibrin due to the stimulation of the fibrinolytic system [22]. Many methods have been used to prevent excessive blood loss after vaginal delivery especially uterotonic agents.
Our study used TA in the third stage of labour for reducing the fibrinolysis process. However, our results demonstrate that the use of TA reduced postpartum blood loss but was not significant in the first two hours postpartum from the placebo group. The reason might be the action of the prophylactic oxytocin in the first two hours. It might have strongly enhanced the contraction of the uterus, so it masked the effect of the TA. However, this result is different from previous studies [16,17].
In the previous studies, Yang H et al., and Gungorduk K et al., reported that the volume of blood loss was decreased significantly after postpartum injection of 1 gm TA [16,17]. However, the Yang H et al., study had some limitations due to the small sample size and randomisation was not clearly mentioned.
Our study’s result is also different from the RCTs about TA in the management of PPH which showed the effectiveness of TA in the treatment of PPH [9, 16-18]. The reason could be, in case of PPH, the primary mechanism of uterine contraction to inhibit blood loss was not enough to prevent postpartum blood loss. Therefore, the TA antifibrinolytic action can help reduce postpartum blood loss. This was different from most vaginal delivery without PPH where uterine contraction can adequately stop the blood losses in a normal situation.
The frequency of PPH was one case in TA group and three cases in placebo group (with one case of severe PPH). More women in the placebo group than in the TA group required additional uterotonic agents but not statistically significant which might be due to the limitation of our sample size.
The strength of our study was the double blind, placebo controlled randomised design and was specified for vaginal delivery group. The dosage of one gram of TA was selected according to data from the previous study so the effect of different dosage of TA needs further evaluation.
Limitation
The sample size was designed to evaluate the difference of blood loss volume between TA and control group, a larger sample size is needed to evaluate the difference of incidence of PPH between both groups. The postpartum haematocrit change was also designed to be measured in this study, however it was removed due to the missing data problem.
Conclusion
The addition of TA in vaginal delivery did not reduce the amount of postpartum blood loss in the first two hours compared to prophylaxis oxytocin only. The data from this study, which has the different results, should be included in the meta analysis with previous and upcoming studies for an accurate guideline on the benefit of TA in prophylaxis of PPH in vaginal delivery.
Disclaimer
None of the authors has any conflict of interest relative to this work. This study did not receive pharmaceutical company support.
Trial Registry number: The study was conducted after approval by the Ethical Research Committee of Udonthani Hospital No.18/2559 and is registered in the Thai Clinical Trial Registry number: TCTR20161104004.
*values are in mean±SD. p-value was calculated by unpaired student t-test
TA: Tranexamic acid
*Adjusted for body weight
†values are in mean±SD. The p-value was calculated by an unpaired student t-test
‡values are in n (%). The p-value was calculated by chi-square and fisher’s exact tests
CI: Confidence interval, TA: Tranexamic acid
*values are in n (%). Using a chi-square and fisher’s exact tests
Total side effect is one case (Nausea)
TA: Tranexamic acid
[1]. Cunningham F, Leveno K, Bloom S, Spong CY, Dashe J, Williams Obstetrics 2014 24eMcgraw-hill [Google Scholar]
[2]. Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, Global causes of maternal death: a WHO systematic analysisThe Lancet Global Health 2014 2(6):e323-e33.10.1016/S2214-109X(14)70227-X [Google Scholar] [CrossRef]
[3]. Cunningham FG LK, Bloom SL, Spong CY, Dasche JSI, Hoffman BL, Casey BM, Sheffield JS, Williams Obstetrics 2014 24th edNew YorkMcGraw-Hill [Google Scholar]
[4]. Sobhy SI, Mohame NA, The effect of early initiation of breast feeding on the amount of vaginal blood loss during the fourth stage of laborThe Journal of the Egyptian Public Health Association 2004 79(1-2):1-12. [Google Scholar]
[5]. World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage: World Health Organization; 2012 [Google Scholar]
[6]. Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, Fergusson DA, Antifibrinolytic use for minimising perioperative allogeneic blood transfusionThe Cochrane Library 2011 10.1002/14651858.CD001886.pub4 [Google Scholar] [CrossRef]
[7]. Buggy F, Sheppard BL, Bonnar J, The effect of tranexamic acid on measured menstrual loss and endometrial fibrinolytic enzymes in dysfunctional uterine bleedingActa obstetricia et gynecologica Scandinavica 1994 73(3):274-77.10.3109/000163494090234538122512 [Google Scholar] [CrossRef] [PubMed]
[8]. Henry J, McFarland A, The effectiveness of tranexamic acid at reducing postoperative blood loss following cesarean section: a systematic review of quantitative evidence protocolJBI database of systematic reviews and implementation reports 2015 13(6):72-81.10.11124/jbisrir-2015-199126455746 [Google Scholar] [CrossRef] [PubMed]
[9]. Nagangouda R, Vinaya G, Tranexamic acid for the treatment of postpartum hemorrhage: placebo controlled studyInternational Journal of Reproduction, Contraception, Obstetrics and Gynecology 2017 6(7):3071-75.10.18203/2320-1770.ijrcog20172937 [Google Scholar] [CrossRef]
[10]. Collaborators WT, Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trialLancet (London, England) 2017 389(10084):210510.1016/S0140-6736(17)30638-4 [Google Scholar] [CrossRef]
[11]. Novikova N, Hofmeyr GJ, Cluver C, Tranexamic acid for preventing postpartum haemorrhageThe Cochrane Library 2015 10.1002/14651858.CD007872.pub3 [Google Scholar] [CrossRef]
[12]. Movafegh A, Eslamian L, Dorabadi A, Effect of intravenous tranexamic acid administration on blood loss during and after cesarean deliveryInternational Journal of Gynecology & Obstetrics 2011 115(3):224-26.10.1016/j.ijgo.2011.07.01521872857 [Google Scholar] [CrossRef] [PubMed]
[13]. Xu J, Gao W, Ju Y, Tranexamic acid for the prevention of postpartum hemorrhage after cesarean section: a double-blind randomization trialArchives of Gynecology and Obstetrics 2013 287(3):463-68.10.1007/s00404-012-2593-y23064441 [Google Scholar] [CrossRef] [PubMed]
[14]. Sentürk MB, Cakmak Y, Yildiz G, Yildiz P, Tranexamic acid for cesarean section: a double-blind, placebo-controlled, randomized clinical trialArchives of Gynecology and Obstetrics 2013 287(4):641-45.10.1007/s00404-012-2624-823143410 [Google Scholar] [CrossRef] [PubMed]
[15]. Lakshmi SD, Abraham R, Role of Prophylactic Tranexamic Acid in Reducing Blood Loss during Elective Caesarean Section: A Randomized Controlled StudyJ Clin Diag Res 2016 10(12):QC1710.7860/JCDR/2016/21702.905028208943 [Google Scholar] [CrossRef] [PubMed]
[16]. Gungorduk K, Asıcıoğlu O, Yıldırım G, Ark C, Tekirdağ Ai, Besımoglu B, Can intravenous injection of tranexamic acid be used in routine practice with active management of the third stage of labor in vaginal delivery? A randomized controlled studyAm J Perinatol 2013 30(05):407-14.10.1055/s-0032-132698623023559 [Google Scholar] [CrossRef] [PubMed]
[17]. Yang H, Zheng S, Shi C, Clinical study on the efficacy of tranexamic acid in reducing postpartum blood lose: a randomized, comparative, multicenter trialZhonghua fu chan ke za zhi 2001 36(10):590-92. [Google Scholar]
[18]. Mirghafourvand M, Mohammad-Alizadeh S, Abbasalizadeh F, Shirdel M, The effect of prophylactic intravenous tranexamic acid on blood loss after vaginal delivery in women at low risk of postpartum haemorrhage: a double blind randomised controlled trialAustralian and New Zealand Journal of Obstetrics and Gynaecology 2015 55(1):53-58.10.1111/ajo.1226225688820 [Google Scholar] [CrossRef] [PubMed]
[19]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019281s030lbl.pdf [Google Scholar]
[20]. Gai MY, Wu LF, Su QF, Tatsumoto K, Clinical observation of blood loss reduced by tranexamic acid during and after caesarian section: a multi-center, randomized trialEur J Obstet Gynecol Reprod Biol 2004 112(2):154-57.10.1016/S0301-2115(03)00287-2 [Google Scholar] [CrossRef]
[21]. Bernard R, Fundamentals of biostatistics 2000 2BostonPWS Publishers:140-246. [Google Scholar]
[22]. As AK, Hagen P, Webb J, Tranexamic acid in the management of postpartum haemorrhageBJOG: An International Journal of Obstetrics & Gynaecology 1996 103(12):1250-51.10.1111/j.1471-0528.1996.tb09638.x8968245 [Google Scholar] [CrossRef] [PubMed]