Infectious diseases are more likely to affect the population all over the world. Hence, antibiotic therapy has become crucial in the effective management of infectious diseases. Antibiotic therapy yield good results when they are administered by IV route. At times, one has to consider the concept of IV to PO conversion of antibiotic therapy. Antibiotics are considered suitable for IV to oral conversion if they have appropriate spectrum, high degree of activity against the presumed or known pathogen, and have good bioavailability. Many patients remain on expensive IV medications, even after they become able to take bioequivalent oral alternatives. Several studies have demonstrated the efficacy and safety of switching from IV to oral antibiotics in clinically stable patients [1,2]. One way of optimising antibiotic use is to switch earlier from IV to oral therapy, with the following advantages: i) benefits to the patient; ii) lower costs and; iii) reduced workload, e.g., reduced incidence of catheter-related infections, a shorter LOHS, a reduction in costs and an associated reduction in workload without sacrificing patient safety [3,4]. Multidisciplinary medical team shall consider the three important factors like proper patient selection, an appropriate therapeutic approach and patient health education for the successful conversion of IV to oral antimicrobial agents [2,5]. Infectious disease specialist shall evaluate the patient and explore the suitability of the patient for IV to oral switch. This eventually may lead to early discharge and reduce cost burden on the patient [6]. Earlier study results report that implementation of pharmacist mediated IV-PO dosage form conversion service found to be more effective in declining the proportion of inappropriate IV doses and associated costs [7].
The IV conversion to PO therapy can reduce length of hospital stay, healthcare costs and risk of complications related to IV access [8,9]. This conversion may be a “switch therapy”, “sequential therapy” or “step-down” therapy. IV to PO switch programs are highly appropriate and more applicable to antibiotics such as fluoroquinolones (levofloxacin, moxifloxacin), tetracyclines (doxycycline, minocycline), macrolides (clindamycin), co-trimoxazole (sulfamethoxazole-trimethoprim), chloramphenicol, linezolid, metronidazole and antifungal drugs such as fluconazole, itraconazole and voriconazole [10]. According to some authorities, approximately 40% of patients starting on IV antibiotics are candidates for a switch to oral antibiotics after 2-3 days of therapy.
There are very few studies on the practice of IV to oral switch in clinical settings of Indian population [11]. Hence, present study aimed to evaluate practice of IV to oral conversion of antibiotics and its impact on length of stay in a tertiary care hospital.
Materials and Methods
Study Design and Ethical Aspects
A prospective observational study was carried out for a period of six months (November 2015 to April 2016) in Dr. Pinnamaneni Siddhartha Institute of Medical Sciences and Research Foundation at Chinaoutpalli, Gannavaram Mandal, Krishna district, Andhra Pradesh, India. It is an 850-bed tertiary care teaching hospital. A total of 667 patients were screened for enrollment. Of which, 117 patients met inclusion criteria and the same were included into the study following obtaining informed consent form. The study protocol was approved by Institutional Ethics Committee of K.V.S.R. Siddhartha College of Pharmaceutical Sciences Institute (Protocol No.: KVSRSCOPS/IEC/2015/007).
Inclusion criteria: i) Adult in-patients receiving an IV antibiotic for more than 24 hours; ii) the patients able to sufficiently absorb oral medications via oral, nasogastric, or feeding tube route; iii) patients in whom signs and symptoms of infection resolving or improving; iv) patients who were improving clinically and stable (negative blood cultures for ≥48 hours; White Blood Cell (WBC) count stable/normalising; A febrile: temperature <100.4°F (38°C) for >24 hour).
Exclusion criteria: Patients younger than 18 years of age; patients who were not eligible for oral formulation based upon a permanent physiologic condition (e.g., malabsorption syndrome); patients with active gastrointestinal bleed; patient refusal of oral medication; patients whose disease severity met the following criteria [ICU vasopressor dependent or haemodynamically unstable, decreased consciousness, seizures, immunocompromised status {neutropenia, Absolute Neutrophil Count (ANC) <1,000 cells/mm3), serious or life threatening infection or disease state that requires the full duration of IV therapy (e.g., CNS infection, bacteraemia, endocarditis, osteomyelitis, septic arthritis, fungemia, endophthalmitis, orbital cellulitis)}].
Study Procedure
A data collection form was prepared and data were recorded by the principle investigator without any interference on results. It was divided into three parts. The first part included demographic characteristics of patients, comorbidities, family history, personal history allergies, primary diagnosis or presumed indication for antibiotic therapy, indication where systemic IV therapy must be maintained, microbiological results if available. The second part includes eligibility of the antibiotic for suitable IV to oral conversion, route of administration, duration of IV therapy, timing and day of conversion of IV to oral therapy, LOHS, antibiotic administered for no indication. Third part includes non converted antibiotics details such as name of the antibiotic administered, route of administration, duration of IV therapy, LOHS and antibiotic administered for no indication. Following data collection, association between “IV to PO conversion” and “LOHS” was assessed using univariate analysis and analysed by Chi-square test. In the study, physician has converted IV to oral antibiotics at his/her discretion based on patient’s clinical stability as defined in inclusion criteria. Pharmacist has simply documented all observations and the data was analysed.
Clinical Outcomes
The principal outcome of this study was to evaluate the practice of the use of IV antibiotic therapy through an assessment of the switch according to inclusion criteria for clinical stability. Secondary outcomes were to evaluate the duration of IV therapy and LOHS, the day and type of conversion of IV to PO therapy.
Statistical Analysis
Categorical data were represented as percentage frequency whereas continuous data were presented as mean±SD. Day of conversion data were analysed using one way ANOVA followed by Tukey’s test while length of hospital stay data were analysed by two way analysis of variance followed by Bonferroni post-hoc test. The tests were considered statistically significant when *p<0.05. Statistical analysis was carried out using Graphpad Prism Software version 5.0.
Results
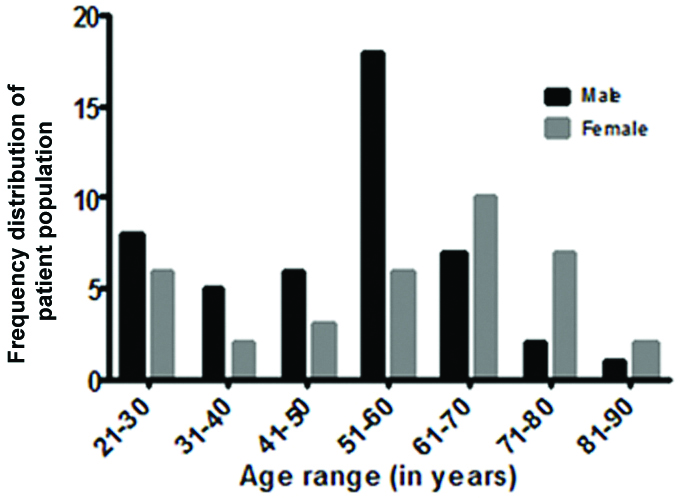
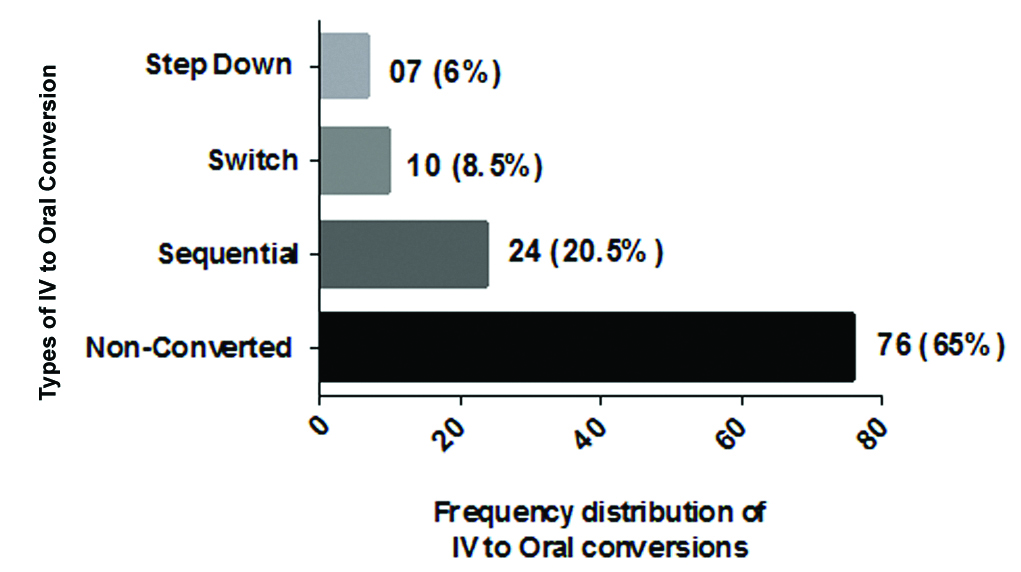
Of the eligible patient population, the higher frequencies of male patients were in the age range of 51-60 while female patients were in the age group of 61-70 years [Table/Fig-1]. Out of 117 patients, “non converted from IV to PO” accounted for 76 (65%) while “converted from IV to PO” accounted for 41 (35%). Out of 41 (100%) of converted, sequential, switch and step-down therapy contributed 24 (58.53%), 10 (24.39%) and 7 (17.07%) respectively. The most frequent type of conversion observed in this study was the sequential conversion therapy [Table/Fig-2].
Age and gender wise distribution of study population.
Frequency distribution of types of IV to oral conversions.
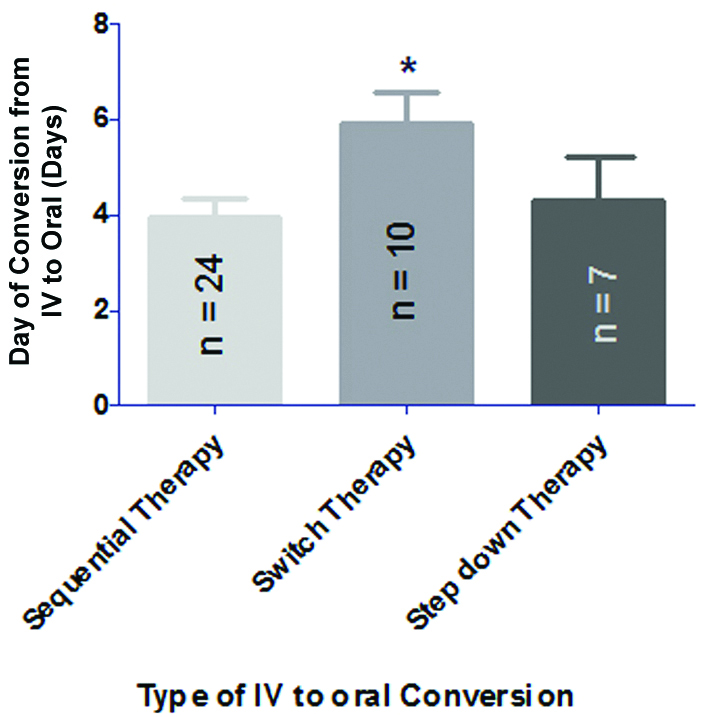
The mean number of days of IV to PO conversion was found to be 3.95±1.73 (mean duration of IV therapy: 3.95 days and mean duration of PO therapy: 2.25 days) in case of sequential conversion whereas the mean number of days of IV to PO conversion in switch and step-down therapy were found to be 5.9±2.02 (mean duration of IV therapy: 5.9 days and mean duration of PO therapy: 2.4 days) and 4.8±2.43 (mean duration of IV therapy: 4.8 days and mean duration of PO therapy: 2.2 days) respectively. Results implied that day of conversion of switch therapy significantly high in comparison to other two conversions [Table/Fig-3].
Day of conversion of IV to oral therapy of antibiotics with respect to various types of IV to oral conversions.
Data are represented as mean±SD and analysed using one way analysis of variance followed by Tukey’s test. *p<0.05 compared to sequential therapy
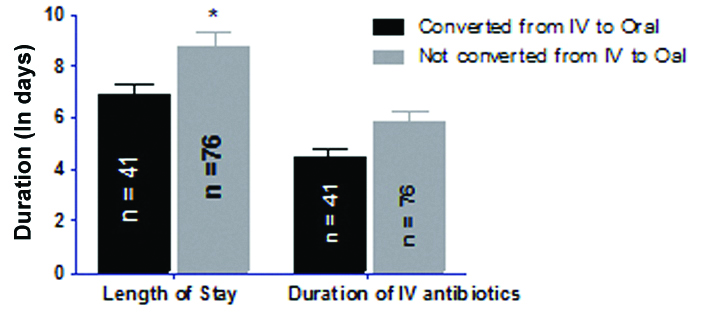
In IV to PO conversion therapy, a mean period of LOHS of patients was 6.84 days (mean duration of IV therapy: 4.48 days and mean duration of PO therapy: 2.36 days) whereas, in case of non converted therapy, mean period of LOHS of patients was 8.71 days (mean duration of IV therapy: 5.86 days). The two-way ANOVA analysis results revealed that there was statistical significant (p<0.05) decrease in LOHS between converted group and non converted group while there is no statistical significant difference between converted group and non converted group with respect to duration of IV antibiotic therapy [Table/Fig-4].
Assessment of association between length of hospital stay and status of conversion from IV to oral therapy.
Data are represented as mean±SD and analysed using two-way analysis of variance followed by Bonferroni post-hoc test. *p<0.05 compared to “converted from IV to oral” group
Discussion
Switching from IV to PO therapy as soon as patients are clinically stable can reduce the length of hospitalisation and lower associated costs. Using the defined criteria for IV to PO switch, 56.32% of the antibiotic sources in the study were not switched to PO therapy, despite improvements in clinical signs of infection. The possible barriers to a timely conversion approach probably are unfamiliarity with guideline recommendations, misconceptions or lack of outcome expectancy [12,13]. In addition, physicians were probably not aware of the existence of clear guidelines on the adequate timing of the conversion. In fact, infection control committee of participating hospitals did not provide these guidelines through booklets or educational sessions.
As far as various patterns of IV to oral are concerned, administered antibiotic courses of treatment that were switched to a suitable oral dosage form were very few and involved antibiotics were mostly macrolides, fluroquinolones and metronidazole classes. These antibiotics were converted in a sequential type to oral formulation. Firstly, these are available in both IV and PO formulations and secondly, sequential therapy is probably easiest way to follow and also due to poor awareness of physicians regarding switch therapy and step-down therapy. On the other hand, it was very rare that cephalosporins switched to oral therapy. Instead, on the day of clinical stability, cephalosporins were discontinued rather than switching to PO therapy. Similar findings were observed earlier study conducted by Hunter KA and Dormaier GK [14]. For instance, drugs such as ceftriaxone with no PO equivalent and hence its conversion to oral dosage form was done using step-down conversion therapy, which was occasionally done in this study.
The mean number of day of IV to PO conversion was found to be 3.95 in case of sequential conversion, correlated with the results of previous studies that also reported the appropriate time for IV therapy to be reassessed between 2-4 days [15,16]. On the contrary, the mean number of days of IV to PO conversion in switch and step-down therapy were found to be 5.9 and 4.8 respectively. The delay in switch therapy and step-down therapy might increase the cost burden on the patient.
Our results revealed that LOHS had significantly (p<0.05) decreased following IV to oral conversion. IV to PO conversion could possibly reduce the hospital stay of patient [3]. On the contrary to the present finding, Shrayteh ZM et al., reported that there is no alteration in the LOHS by using IV to PO conversion strategy whereas duration of antibiotics is significantly reduced [17]. By contrast, in the present study, duration of IV antibiotics has not been significantly decreased following IV to oral conversions.
Limitation
The major limitation in present study was that univariate analysis may not take all the confounding factors that associate with LOHS into consideration. Multivariate logistic regression analysis could be a better approach to identify other confounding factors (other than IV to PO conversion) that associate with outcomes like LOHS and duration of antibiotics. In addition, pharmacist intervention has not provided in the study. In order to fill these gaps, another study needs to be conducted with larger sample size.
Conclusion
Timely and appropriate switching of antibiotics from IV to oral therapy could reduce the length of hospitalisation of patients.