Infantile tremor syndrome is a self-limiting clinical state of unknown aetiology. ITS is characterised by tremors, anaemia, pigmentary skin changes, regression of developmental milestones, and muscle hypotonia, in a plump looking child [1]. The tremors in ITS are coarse in character, and decrease or disappear in sleep [2]. Non specific changes have been described in ITS on neuroimaging in the form of cerebral atrophy [3]. However, changes of cerebral atrophy are also seen in the cases of malnutrition and after certain viral infections of central nervous system [3]. In the past, pneumoencephalography showed evidence of ventricular dilatation and cortical atrophy in patients of ITS [4]. Mathur GP et al., have suggested that the above anatomical changes might be solely due to the result of Protein Energy Malnutrition (PEM) [5]. A case report of ITS from India reported findings from Computerised Tomography (CT) scan of the brain showing cerebral atrophy with thinning of corpus callosum [6].
Cranial neuroimaging has been an integral part of investigations for establishing aetiological correlation in differential diagnosis of conditions associated with tremors. Tremors have been classified as physiologic, enhanced physiologic, essential tremor syndrome, dystonic, Parkinsonian, cerebellar, Holmes, palatal, neuropathic tremor syndrome, drug induced and toxic tremors, psychogenic tremors [7] with various neuroimaging findings. In a rare case report of Rubral (Holmes) tremors cranial Magnetic Resonance Imaging (MRI) revealed ischaemic lesions in thalami bilaterally [8]. In another study on patients with Essential Tremors (ETs) high resolution proton density and T2 weighted MRI images of 12 patients and 15 control subjects showed no structural abnormalities of the brain [9]. Various degrees of cerebral atrophy have been evaluated in children having central nervous system manifestations of PEM [10]. In a case report of childhood head tremors, MRI showed no abnormal findings [11]. Hypomyelination disorders, which sometimes present with extrapyramidal movements, have displayed cerebral and cerebellar atrophy and little or no myelin in the cerebral white matter on MRI scans of most adolescents and adults [12]. The infantile form of Pelizaeus-Merzbacher Disease (PMD) presenting with head tremors, revealed mild to moderate brain atrophy [13]. However, there is still a dearth of MRI data on the patients clinically diagnosed as ITS. Therefore, we set out to identify pertinent MRI markers of ITS through a prospective analysis of clinical examination and cranial MRI scans.
Materials and Methods
This observational cross-sectional study was done among consecutive patients clinically diagnosed as having ITS and who were admitted in the Department of Paediatrics, RD Gardi medical college, Ujjain, Madhya Pradesh, India during the period of April 2013 to August 2014. Cases were included in the study based on clinical definition of ITS having tremors involving extremities, head and tongue with tremulous voice which disappear on sleep [1]. Children with congenital anomalies, head trauma, tumors, sick children with unstable vitals and children whose parents were not willing to be part of study were not included. A predesigned proforma was filled for all patients included in the study.
The proforma included: complete patient profile, general and systemic examination findings and findings of MRI of the brain. MRI scanner was used for doing T1 Weighted spin echo (T1W)-axial, T2 Weighted fast spin echo (T2W)-axial and sagittal Fluid Attenuation Inversion Recovery (FLAIR)-axial plane. A Gradient Recall Echo (GRE) T2* coronal diffusion weighted sequence was done as an optional sequence. The technical details are as follows: machine make GE (GE healthcare, Milwaukee USA) HDe model 8 channels 1.5 Tesla MRI scanner. The coil that was used for scanning in the study was 8 channels NV head neck coil. The sequences done for patients were T2 weighted (TR/TE/ Flip=6200/104/90, FOV 24×24, Matrix 320×224, NEX 1 and Slice thickness 5 mm spacing 1 mm), T1 weighted (TR/TE/ Flip=580/15/80 FOV 24×24, Matrix 288×224, NEX 1, Slice thickness 5 mm and spacing 1 mm) and Flair (TR/TE/ Flip=8100/85/90, TI 2000 FOV 24×24, Matrix 320 × 224, NEX 1 Slice thickness 6 mm and spacing 1 mm) (TR and TE in milliseconds and Flip angle in degrees). Anthropometric evaluation of ITS cases done on the basis of WHO classification of Undernutrition [14]. All PEM patients received treatment as per the WHO guidelines for undernourished children [15]. A written informed consent was taken from the parents of all study subjects. The Institutional Ethics Committee approved this study.
Results
A total of ten patients were admitted with clinical diagnosis of ITS during the study period who were then included in the study. Four were males and six females. Mothers of all study subjects were vegetarian. All children belonged to rural areas and were Hindu by religion. The anthropometric analysis revealed that four patients had severe wasting, three had moderate wasting and one each had severe and moderate stunting. Only one case had microcephaly i.e., head circumference below-3 SD for the age and sex [Table/Fig-1].
Showing anthropometric findings of ITS cases based on WHO classification of Undernutrition [14].
| ITS Case S.No. | Weight for height (%)* | Height for age (%)** | Head circumference (SD) |
|---|
| 1 | 80-90 | <85 | < -2 |
| 2 | 80-90 | 90-95 | < -2 |
| 3 | <70 | >95 | < -1 |
| 4 | 70-79 | 85-89 | < -1 |
| 5 | <70 | >95 | < -1 |
| 6 | <70 | >95 | < -3 |
| 7 | 70-79 | >95 | < -1 |
| 8 | 80-90 | >95 | > -1 |
| 9 | <70 | 90-95 | > -1 |
| 10 | 70-79 | 90-95 | < -1 |
*Weight for Height: Moderate wasting = 70-79%, Severe wasting = <70%
**Height for Age: Moderate stunting = 85-89%, Severe stunting = < 85%
Non specific cerebral atrophy of different grades was observed in all cases except one. In [Table/Fig-2], reduced thickness of corpus callosum was observed in six patients on MRI brain studies. ITS cases who had severe wasting, three out of four showed evidence of cerebral atrophy; all three children with moderate wasting had evidence of cerebral atrophy. Cerebral atrophy was observed in all children with stunting as well as in children having normal height for age and sex. Some patients (6/10) had mild to moderate ventricular dilatation and most patients (9/10) had prominence of Sylvian sulcus. Ventricular dilatation and prominence of Sylvian sulcus had correlation with the grades of cerebral atrophy in all cases [Table/Fig-3,4,5,6,7,8,9,10,11 and 12].
Showing MRI brain findings in ITS cases (1-10 in sequence of case serial numbers).
| ITS case S. No. | Age (months) | Sex | Cerebral atrophy | Cerebellar atrophy | Ventricular enlargement | Sylvian sulcus prominence | Corpus callosum |
|---|
| 1 | 12 | F | Mild, diffuse, marginally poor myelination | Nil | Nil | Less prominent | Reduced thickness |
| 2 | 10 | M | Severe, diffuse | Nil | Severe | Very prominent | Reduced thickness |
| 3 | 24 | M | Nil | Nil | Nil | Normal | Normal |
| 4 | 11 | F | Severe, diffuse | Mild cerebellar atrophy | Severe | Very prominent | Reduced thickness |
| 5 | 5 | M | Severe, mainly temporal and frontal lobes | Nil | Nil | Prominent | Reduced thickness |
| 6 | 7 | F | Mild | Nil | Nil | Less prominent | Normal |
| 7 | 8 | F | Severe, diffuse | Nil | Mild | Prominent | Reduced thickness |
| 8 | 9 | M | Severe, predominantly fronto-temporal lobes | Nil | Mild | Prominent | Normal |
| 9 | 13 | F | Severe, diffuse predominantly fronto-temporal lobes | Nil | Moderate | Prominent | Normal |
| 10 | 8 | F | Severe, predominantly fronto-temporal, marginally poor myelination, predominantly peritrigonal white matter | Nil | Moderate | Prominent | Reduced thickness |
T2W sagittal, axial FLAIR and T2W sequence revealing diffuse atrophy and reduced volume of corpus callosum. Hyperintensity on T2W sequence suggestive of marginally poor myelination.
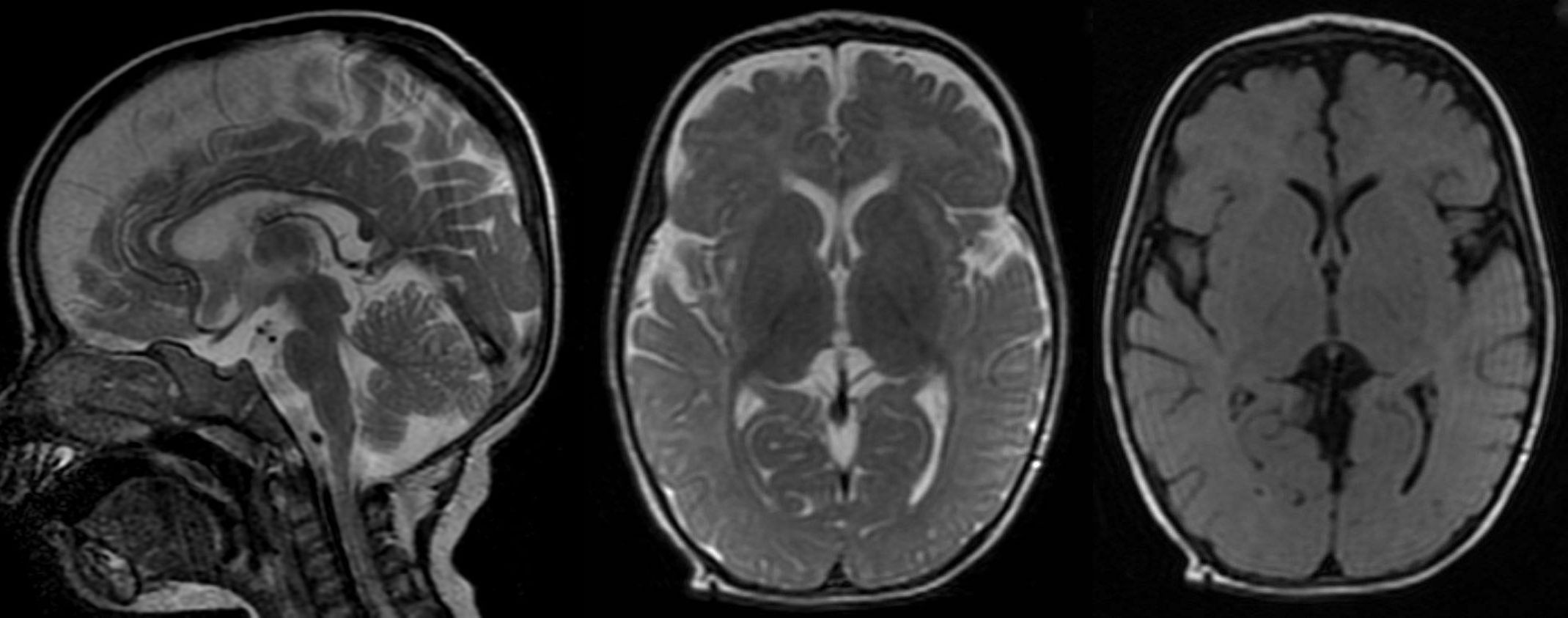
T2W sagittal, axial and axial FLAIR sequence showing significant diffuse atrophy and reduced volume of corpus callosum. No evidence of poor myelination appreciable.
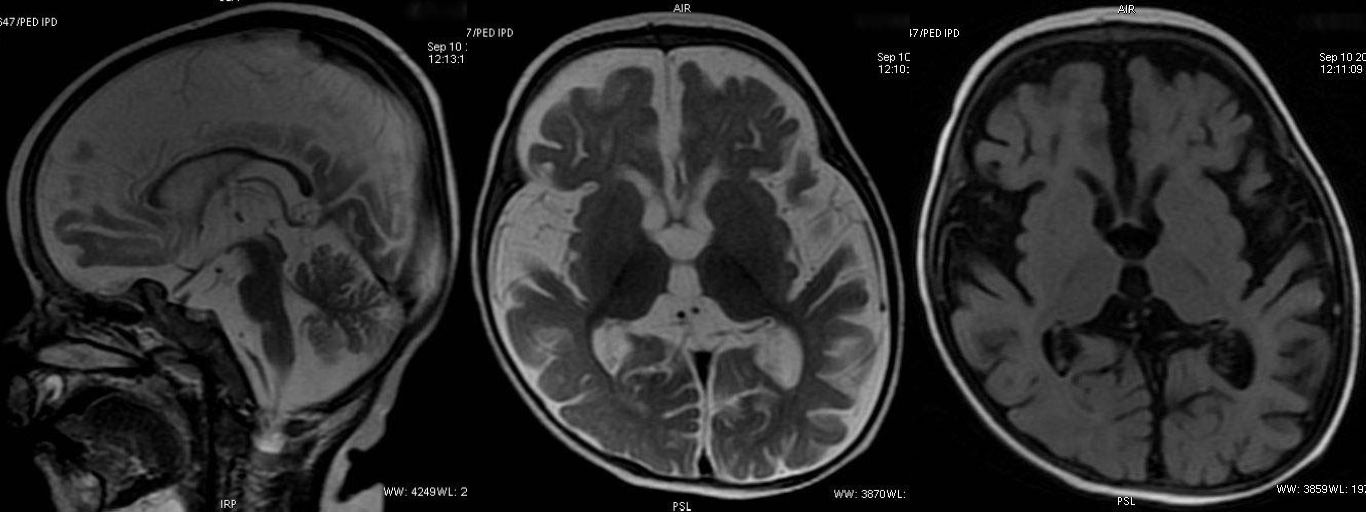
T2W sagittal, axial FLAIR and T2W axial sequence revealing normal appearance of brain.
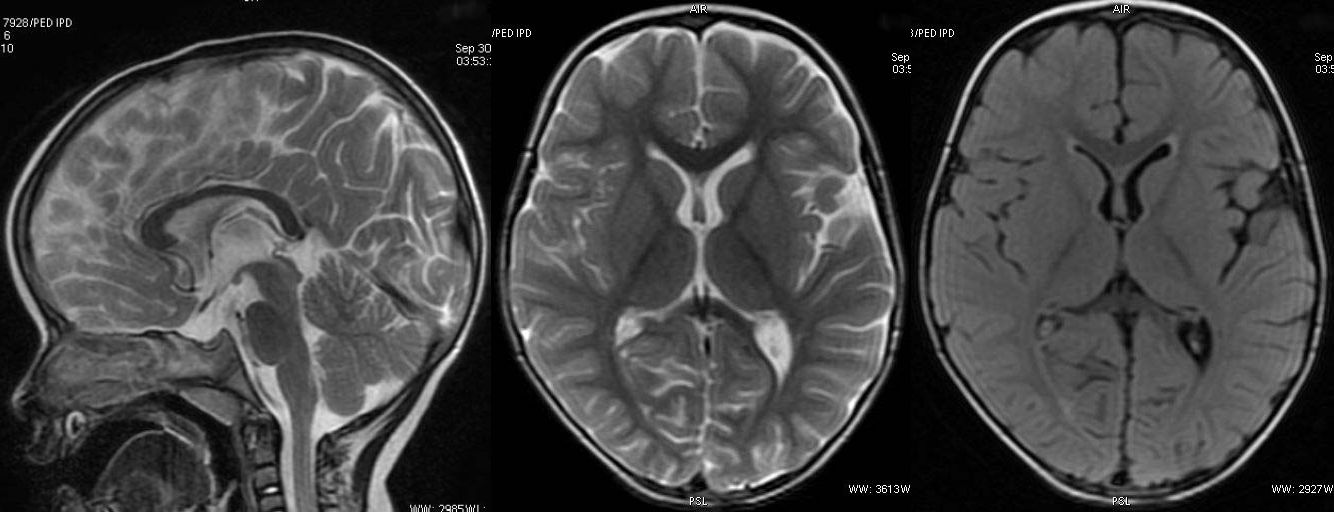
T2W axial, sagittal and axial FLAIR sequence revealing significant diffuse atrophy and reduced volume of corpus callosum. Prominence of sylvian fissure and CSF spaces noted. Mild cerebellar atrophy is appreciable in sagittal T2W image.
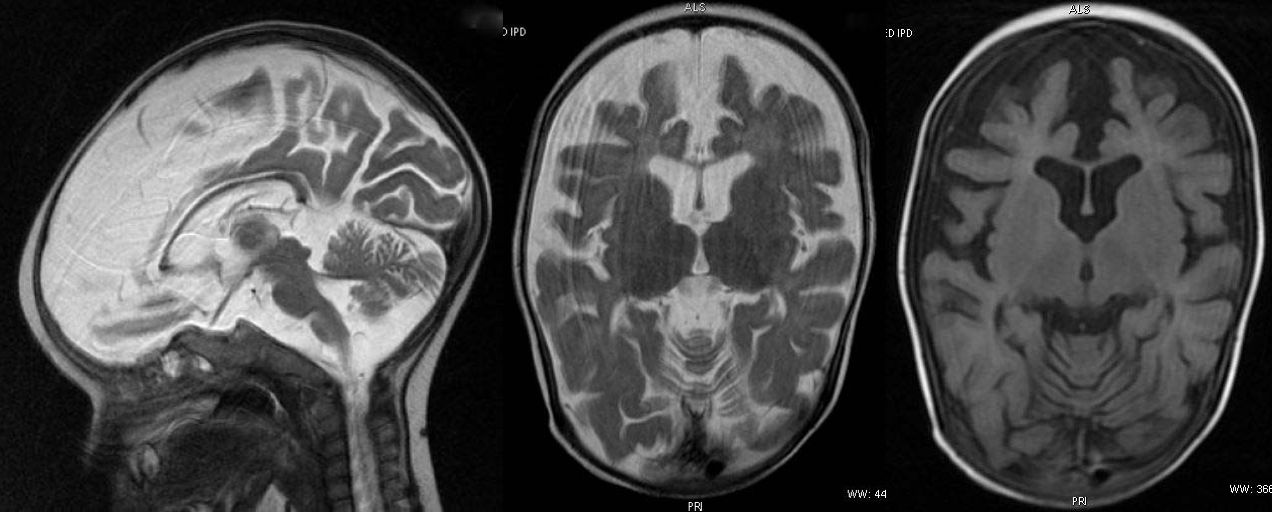
T2W axial, sagittal and axial FLAIR sequence revealing significant atrophy involving the frontal and temporal lobes and reduced volume of corpus callosum. Faint T2W hyperintensity of white matter is compatible with age.
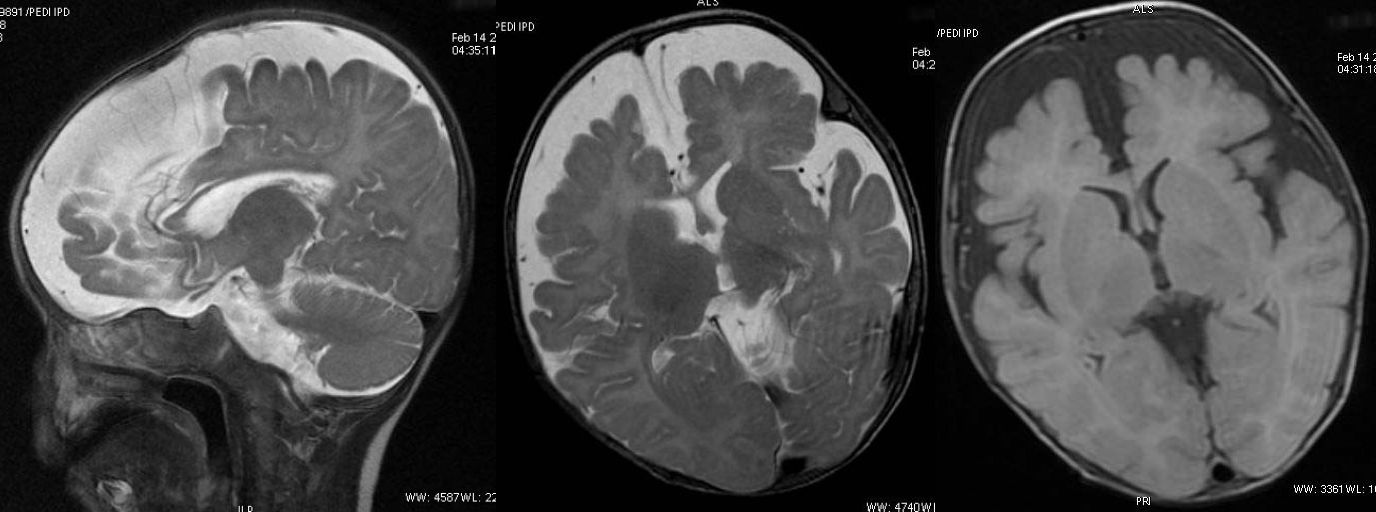
T2W axial, sagittal and axial FLAIR sequence revealing mild cerebral atrophy and normal corpus callosum.
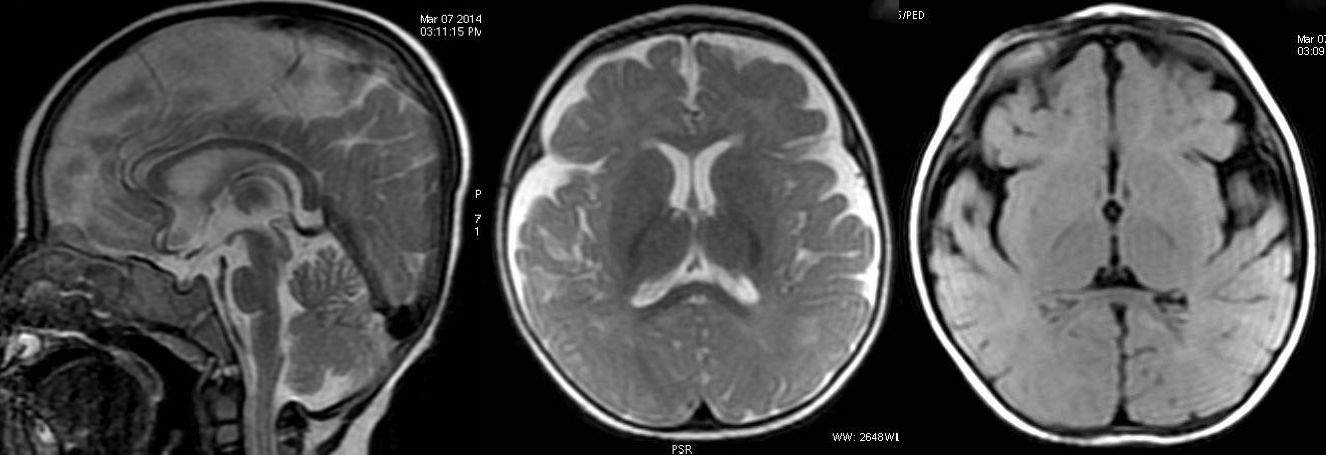
T2W axial, sagittal and axial FLAIR sequence revealing significant atrophy and reduced volume of corpus callosum.
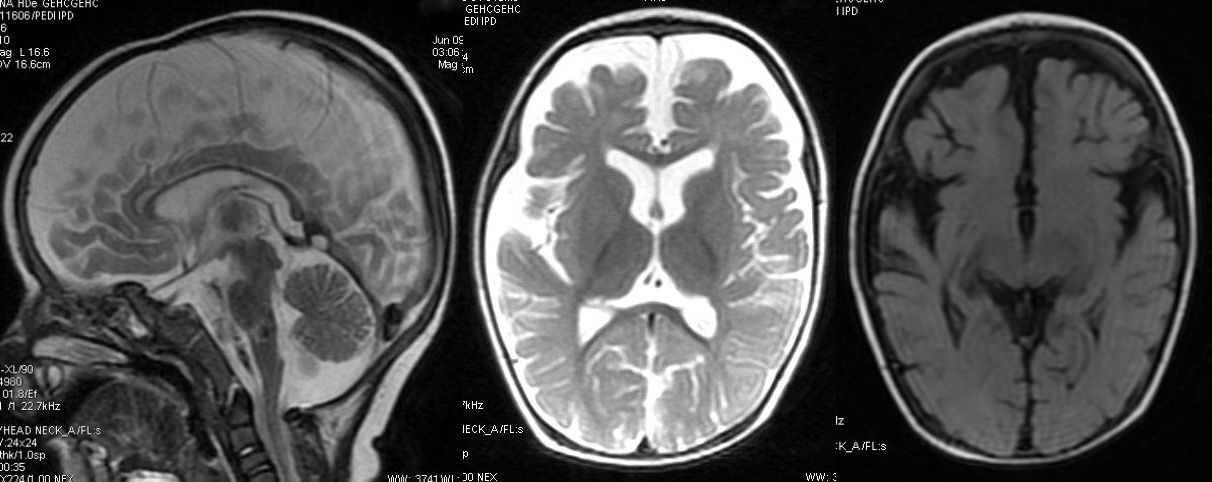
T2W and axial FLAIR sequence revealing significant atrophy involving the frontal and temporal lobes.
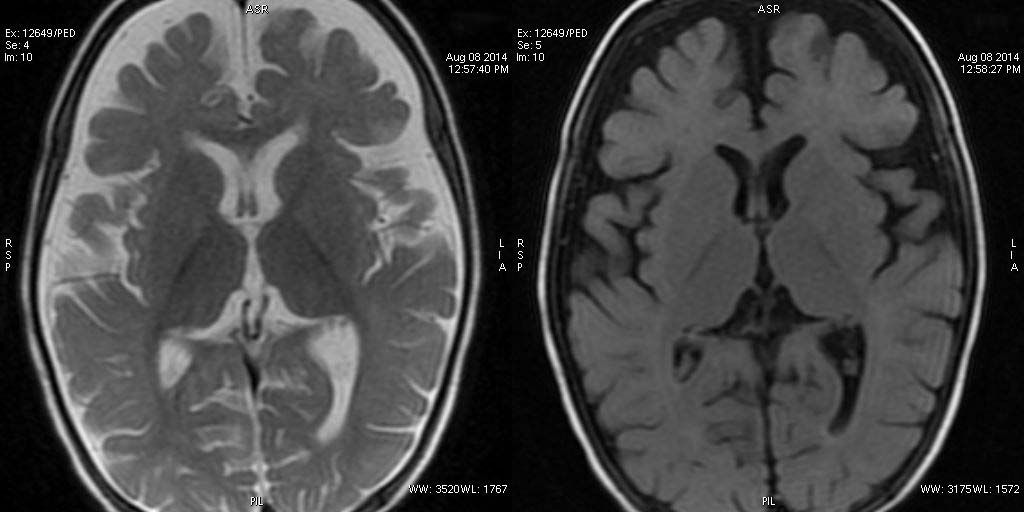
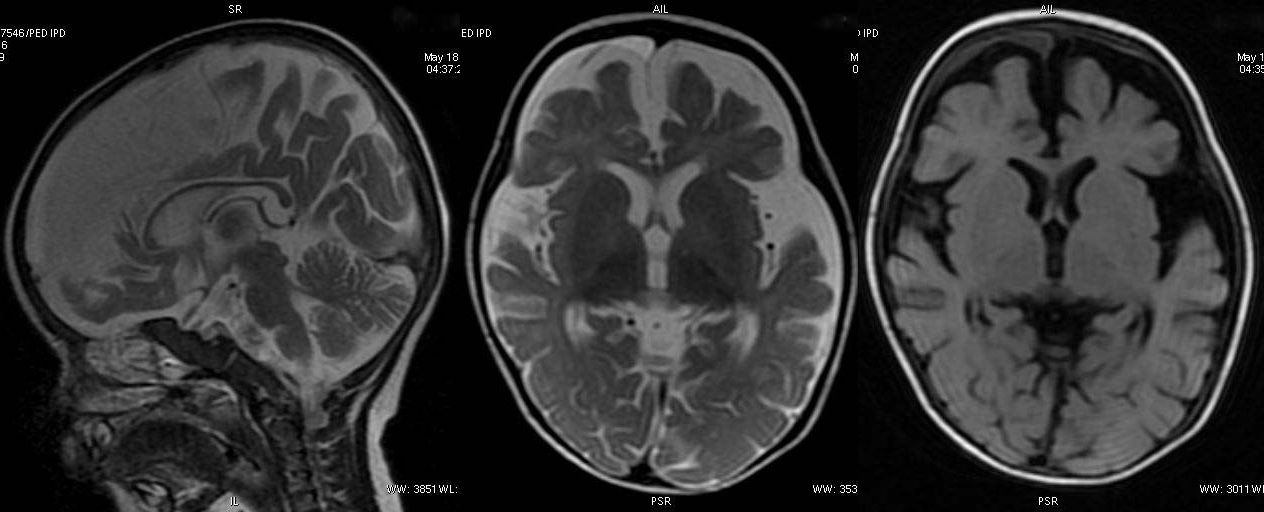
T2W axial, sagittal and axial FLAIR sequence revealing significant atrophy involving the frontal and temporal lobes. The appearance of corpus callosum is within normal limits.
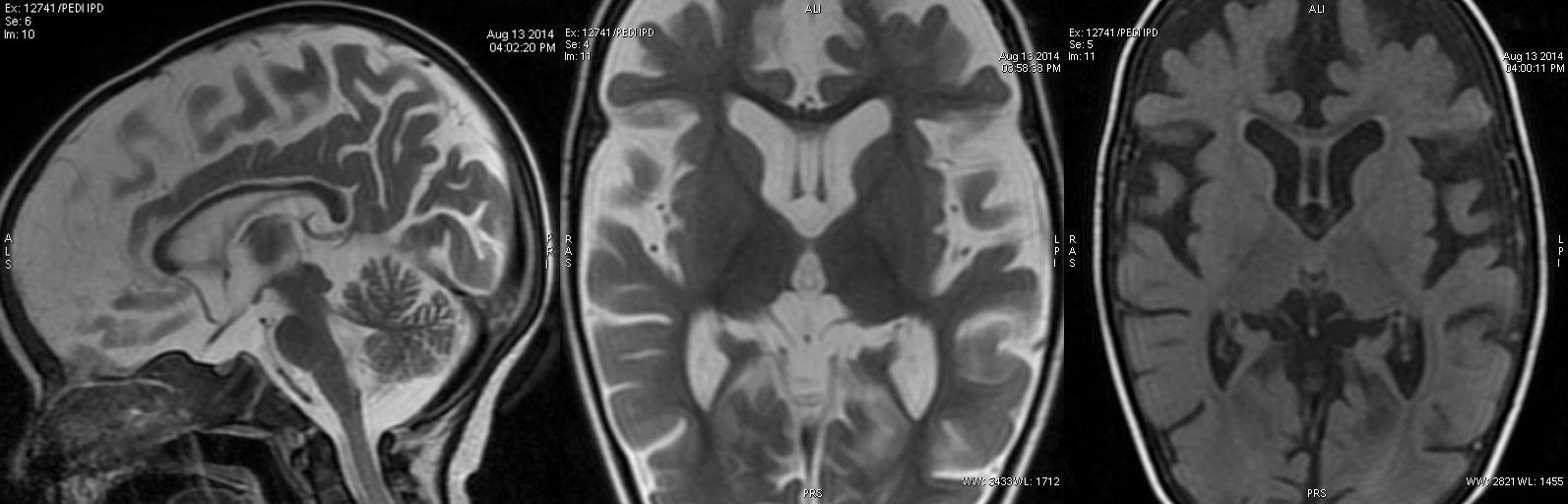
T2W axial, sagittal and axial FLAIR sequence revealing significant atrophy predominantly involving the frontal and temporal lobes and reduced volume of corpus callosum. Also, noted marginally poor myelination, predominantly peritrigonal white matter.
Discussion
Thora S et al., and Mathur GP et al., observed that ITS has been thought to be occurring mainly due to the result of protein calorie malnutrition [3,5]; however, some researchers hypothesised different aetiologies: slow viral infections like Kuru and Scrapie [16], a possibility of a metabolic factor such as transient enzyme defect in tyrosine metabolism [1], Vitamin B12 deficiency [17] and Zinc deficiency [18].
Neuroimaging was limited to pneumoencephalography in the past [4] but with recent advances in radio diagnostic imaging, studies have come up with neuroimages in ITS cases [3,6,18] and revealed non specific diffuse cerebral atrophy. This study also depicted different grades of cerebral atrophy along with reduced thickness of corpus callosum representing loss of white matter. Presence of ventricular enlargements in many patients in the present study also lends support to presence of cerebral atrophy.
Childhood tremors have a vast spectrum of aetiology. Tremors have been classified on the basis of distinction between rest, postural, simple kinetic and intention tremors [7]. Essential tremor is a common form of tremor in children, most often seen in teenagers. Other tremors in children are due to underlying metabolic, endocrine, or heredodegenerative disorders or the effects of various drugs [19]. However, in one study no identifiable pathology was detected in 20 studied cases of essential tremors even after autopsy [20]. Rubral tremor, rare type of tremor characteristically presents at rest, cranial MRI revealed ischaemic lesions in thalami bilaterally in two cases but failed to reveal any mesencephalic lesion [8]. Rubral tremor tended to disappear during sleep, and increased in amplitude on awakening and in response to verbal, tactile and noxious stimuli [8]. These characteristics of the Rubral tremors are similar to that of tremors in ITS cases [21]. However, unlike Rubral tremors no thalamic lesions were observed in the present study in cases of ITS.
ITS has been shown to be associated with various nutritional deficiencies [17,18] many of which are present in patients of PEM [5]. Cerebral atrophy in infants with PEM has been observed, however the proportion of ITS in PEM patients has not been mentioned [10]. The main reason is that as the human brain grows rapidly from the 11th week of gestation to the second year of life, malnutrition during this period would be expected to impair the growth and functioning of the brain [10]. Present study has shown different grades of cerebral atrophy in the ITS cases, having moderate (three) to severe (four) wasting. Thus, our results also favoured nutritional aetiology for ITS. Our research group had previously demonstrated reversibility of cerebral atrophy using MRI scans done at the time of presentation and on follow up of six to eighteen month period in three ITS cases [22]. The observations of reversibility of cerebral atrophy are similar to reversible cerebral changes in malnourished children [23,24]. This supports nutritional factors in the aetiology of ITS but no specific nutritional factor could be identified so far.
In Ramakumar L et al., study, an unusual finding was observed, 4 out of 29 of the nerve biopsies revealed partial demyelination and swelling of the myelin sheath in ITS cases [25]. One study revealed that early cerebellar atrophy is indicated by homogeneity of the white matter signal; and lesions in deep white matter on T2-weighted images, other changes in hypomyelinating disorders included abnormal signal intensity in basal ganglia and pons [12].
PMD a disorder of cerebral myelination with variable phenotypes (infantile form) presents with muscular hypotonia, nystagmus, head tremor and slowly progressive extrapyramidal and pyramidal symptoms with survival to adolescence or young adulthood [13]. Two patients with PMD had been described with an apparent loss of white matter after 15 years of age. This white matter loss was simultaneously associated with severe global atrophy, severe corpus callosum atrophy, and a mild increase in global and cerebellar atrophy over time [26]. In the present study, cerebellar atrophy has been identified in only one child with ITS [Table/Fig-2] having shrunken folia and large cerebellar fissures with volume loss. Cerebellar atrophy is a relatively common, but non specific finding in children due to extreme prematurity, neonatal hypoxic ischaemic encephalopathy, post inflammatory, vitamin B12 deficiency, toxins (Phenytoin and other anti epileptic drugs, tacrolimus), paraneoplastic, radiation therapy [27]. Further studies are required for aetiological considerations of cerebellar atrophy in ITS patients.
An association of cerebral atrophy with thin corpus callosum has also been described in CT scan of the brain with ITS in a 5.5-month-old infant [6]. In the present study reduced thickness of corpus callosum has been observed suggesting white matter involvement similar to above studies.
Till now this could be established that ITS is solely a manifestation of nutritional disorder but there is still a dilemma why only few children with undernutrition present with tremors. These findings of neuroimaging will support further research to identify specific factor responsible at molecular level that causes tremors in ITS.
Limitation
Our study had certain limitations i.e., we tried to evaluate extent of cerebral atrophy using neuroimaging techniques [28] but, in the absence of universally applicable standard guidelines for defining the extent of cerebral atrophy we could not present our findings objectively on a scale. Association of grades of cerebral atrophy with intensity and duration of tremors was also not studied in the present study.
Conclusion
Cranial neuroimaging in tremor patients have wide spectrum of unveiling illustrations. ITS patients have shown different grades of cerebral atrophy, ventricular enhancement along with corpus callosum thinning. These finding support a nutritional deficiency as the main aetiology for ITS for this study. There is need for more studies on clinico-neuroimaging correlation in patients of ITS. Further, case control studies can throw light on the causation of ITS by identifying specific factors that lead to development of tremors in only a few undernourished children.
Author’s Contribution
RG, AP and JM: involved in the study concept, designing and manuscript writing; RG: did assessment of patients; PG: did radiological analysis; RG, PS: did literature search. All the authors approved the final manuscript. AP will act as the guarantor of the study.
*Weight for Height: Moderate wasting = 70-79%, Severe wasting = <70%
**Height for Age: Moderate stunting = 85-89%, Severe stunting = < 85%