A Rare Case of Amphotericin B Resistant Cryptococcal Meningitis in a HIV Non-reactive Immunocompetent Patient
Pulin Kumar Gupta1, Subodh Kumar Mahto2, Brinder Mohan Singh Lamba3, Mahinder Pal Singh Chawla4, Ankita Sheoran5
1 Professor, Department of Medicine, DR. RML Hospital and PGIMER, New Delhi, India.
2 Senior Resident, Department of Medicine, DR. RML Hospital and PGIMER, New Delhi, India.
3 Professor, Department of Medicine, DR. RML Hospital and PGIMER, New Delhi, India.
4 Professor, Department of Medicine, DR. RML Hospital and PGIMER, New Delhi, India.
5 Post Graduate, Department of Medicine, DR. RML Hospital and PGIMER, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Subodh Kumar Mahto, Senior Resident, Medicine Department, OPD Block, DR. RML Hospital, New Delhi-110001, India.
E-mail: drsubodhkr05@gmail.com
Cryptococcus meningitis is the most common opportunistic fungal infection in immunocompromised individuals and rarely occurs in HIV non-reactive people. Fever, headache, altered sensorium and neck stiffness are the usual presenting features of meningitis and common causative agents are of viral, mycobacterial and mostly bacterial origin in an immunocompetent host. However, fungal infection should be considered amongst the differentials. Knowledge of geographical distribution and a high degree of clinical suspicion are important for an early diagnosis, institution of early treatment and ultimately preventing lethal complications. We hereby report a case of amphotericin B resistant cryptococcal meningitis in a HIV non-reactive immunocompetent individual.
Brain, Fungal infection, Glasgow coma scale, Obstructive hydrocephalus, Pleocytosis
Case Report
A 52-year-old female, housewife, was admitted in the Department of Medicine in our hospital, with chief complaints of fever associated with headache for three weeks. She had multiple episodes of vomiting and an altered sensorium since the last two days. Fever was intermittent, of high grade (103°F), associated with chills and rigors and was relieved by taking antipyretics. It was associated with constant headache and vomiting. Vomiting was projectile in nature, multiple episodes, non-bilious, non-bloody and not relieved by taking antiemetics. Patient developed an altered sensorium two days prior to admission. She had no history of seizures, head trauma, ear discharge or drug abuse. There was no significant past medical or surgical history. On examination, the patient was unconscious and not oriented to time, place and person with a Glasgow Coma Scale (GCS) of 10/15. Her core temperature was 103° F. Her pulse rate was 110 beats/minute, regular and blood pressure was normal in all four limbs. Central Nervous System (CNS) examination revealed no neck rigidity. All cranial nerves were normal and deep tendon reflexes were diminished. Bilateral pupils were normal and reactive to light and bilateral planters were unelicitable. Examination of other systems revealed no obvious abnormality and fundus examination was normal.
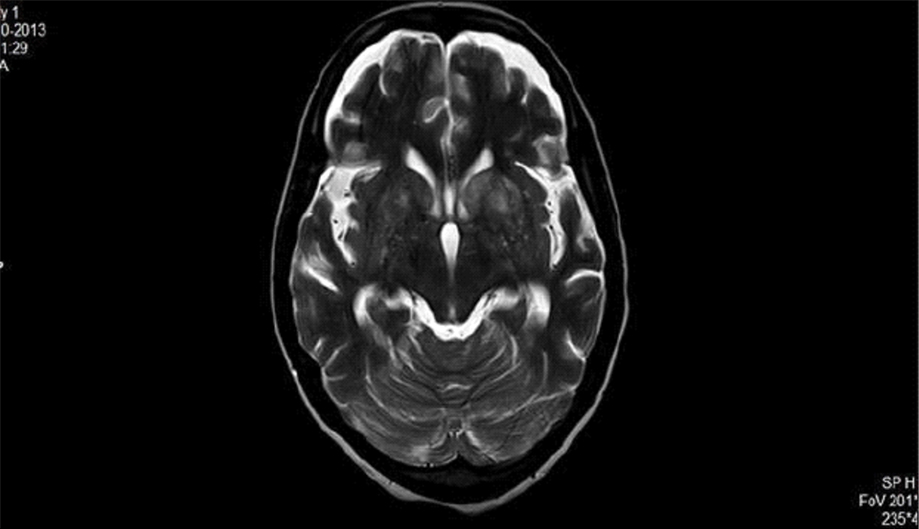
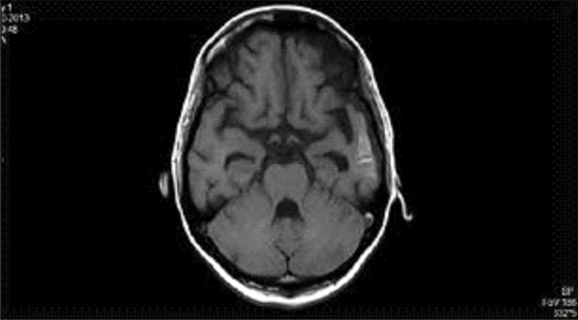
Laboratory tests revealed 12 gm/dL hemoglobin and Total Leucocyte Count (TLC) of 7,900/mm3 (with 66% neutrophils, 30% lymphocytes and 4% eosinophils) and platelet count of 250000/mm3. The Erythrocyte Sedimentation Rate (ESR) was 15 mm/first hour. Urine routine and microscopy, liver function tests and renal function tests were all within normal limits. HBsAg, Anti HCV and ELISA for HIV were all non-reactive. Salmonella typhi IgM and IgG were negative. Blood and urine cultures were sterile. Malaria antigen test was negative for both P. vivax and P. falciparum. Dengue serology (IgM and IgG), NS1 antigen and chikungunya serology were negative. Non-Contrast Computerized Tomography (NCCT) of head done revealed obstructive hydrocephalus. A guarded Lumbar Puncture (LP) was done. Cerebrospinal Fluid (CSF) opening pressure was high. CSF examination revealed cells-mononuclear pleocytosis, with 74 mg/dL sugar and 102 mg/dL protein. FLAIR T2 of brain showing cryptococcomas in basal ganglia while Contrasted Enhanced Magnetic Resonance Imaging (CEMRI) brain T1 weighted image showing temporal horn dilation suggestive of obstructive hydrocephalous [Table/Fig-1,2].
FLAIR T2 of brain showing cryptococcomas in basal ganglia.
CEMRI of brain T1 weighted showing temporal horn dilatation s/o obstructive hydrocephalus.
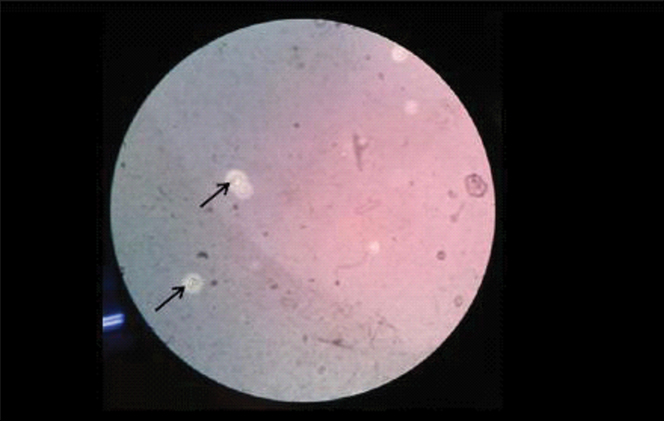
CSF staining by India ink was done and budding the yeast cells with a halo, suggestive of Cryptococcus like organisms were seen. [Table/Fig-3] CSF for viral serology for Herpes simplex virus, Japanese encephalitis, Varicella zoster virus was negative. Similarly, CSF staining by Giemsa and Ziehl Nielsen stain was negative.
Showing budding yeast cells with surrounding holes. (Negative India ink staining, 100X).
CSF culture on Sabouraud’s Dextrose Agar (SDA) yielded smooth colonies of yeast after five days of incubation at 37° C. The urease test of this isolate was positive. The isolate was subcultured on Canavanine Glycerine Bromothymol Blue (CGB) to differentiate C. neoformans from C. gattii and found to be positive for C. neoformans. Cryptococcal antigen was positive in CSF (titre-1:16). Since the HIV status was negative, so we asked for immunological profile of the patient. Her CD4 count was 239/mm3 (Normal range: 500-1500/mm3) and the levels of IgA (85 mg/dL), IgM (216 mg/dL) and IgG (878 mg/dL) were all within normal limit.
Patient was initiated on amphotericin B (1 mg/kg/day, IV) and flucytosine (100 mg/kg/day, orally) and was continued for four weeks. There was minimal but definite improvement in her clinical status. A repeat CSF examination after four weeks of treatment turned out to be positive for Cryptococcus neoformans (India ink and CSF antigen). Treatment with amphotericin B (1 mg/kg/day) and flucytosine (100 mg/kg/day) was continued further for two weeks, keeping a check on her electrolytes and kidney function tests.
CSF samples for culture and an in vitro antibiotic susceptibility were sent and found to be positive for Cryptococcus neoformans but resistant to amphotericin B and sensitive to flucytosine, fluconazole and oriconazole. CD4 counts were repeated after six weeks of treatment and were 629/mm3 (improved). The initial low values maybe because of the invasive fungal infection per se.
Patient was kept in isolation. She was started onfluconazole 800 mg/kg/day along with supportive management. Maintenance of oral hygiene along with chlorhexidine body wash was given to her by hospital staff on daily basis.
The patient gradually improved clinically and her headache, fever, vomiting, was relieved but she did not give consent for a repeat LP and so the patient was discharged on request, on fluconazole 800 mg/kg/day. Her liver function tests, kidney function tests were within normal limits at all times. Repeat MRI of brain was still suggestive of obstructive hydrocephalus although, reduced to some extent when compared with the previous one.
Discussion
Cryptococcus neoformans, an encapsulated saprophytic, ubiquitous environmental fungus, is a cause of common opportunistic infection in immunocompromised individual [1]. Globally, there are about 1 million cases diagnosed each year and 600,000 deaths per year [1,2]. However, cryptococcal infection in immunocompetent host is not an uncommon entity and frequently and easily misdiagnosed as viral or tubercular meningitis [3].
Globally, capsular serotype D (C. neoformans var. neoformans) is the most common and causes 82% of cryptococcal disease [4]. While in northern Europe, 20%-30% of HIV associated Cryptococcus meningitis is caused by capsular serotype A (C. neoformansvar. grubii) [5].
As the antigen detection test for Cryptococcus is highly accurate for the diagnosis of its invasive nature, both serum and CSF are used for detection of cryptococcal polysaccharide antigen [6].
According to the Infection Disease Society of America (IDSA) guidelines, the combination of amphotericin B (1 mg/kg/day) and flucytosine (100 mg/kg/day) is recommended for the induction phase of treatment. In severe meningitis, flucytosine can be added to amphotericin B which helps to decrease the rates of treatment failure [7,8]. Fluconazole can be used in combination with amphotericin B at 800 mg/kg/day dosing for induction when flucytosine is not available [9].
Amphotericin B with or without flucytosine are used for treatment of cryptococcal meningitis in non-HIV infected patients for four weeks in induction phase. Patients then can receive fluconazole at 400-800 mg/kg/day for eight weeks for the consolidation phase which is further decreased to 200 mg/kg/day for the suppressive phase. The treatment can be stopped once the patient starts improving symptomatically i.e. minimum of two CSF cultures are negative with normal glucose level, or it can also be stopped after six months to one year of being asymptomatic [10].
To our knowledge, this is probably the first reported case of amphotericin B resistant cryptococcal meningitis in a HIV non-reactive immunocompetent patient from North India.
Conclusion
We hereby present this case to sensitize the clinicians towards the emerging amphotericin resistant cryptoccocal meningitis which is increasingly encountered in HIV and as well as HIV non-reactive immunocompetent hosts. Amphotericin B in combination with flucytosine, as compared with amphotericin B alone, is associated with improved survival among patients with cryptococcal meningitis. A survival benefit of amphotericin B with fluconazole was not found.
[1]. Lin YY, Shiau S, Fang CT, Risk factors forinvasive Cryptococcus neoformans diseases: a case-control studyPLoS One 2015 10:e011909010.1371/journal.pone.011909025747471 [Google Scholar] [CrossRef] [PubMed]
[2]. Panackal AA, Wuest SC, Lin YC, Wu T, Zhang N, Kosa P, Paradoxical Immune Responsesin Non-HIV Cryptococcal MeningitisPloSPathog 2015 11:e100488410.1371/journal.ppat.100488426020932 [Google Scholar] [CrossRef] [PubMed]
[3]. Louro R, Ferreira R, Pinheiro C, Parada H, Faria D, Monteiro E, Fungal meningitis in an immunocompetent patientClin Drug Investig 2013 33(Suppl 1):S 47-50.10.1007/s40261-012-0021-523381985 [Google Scholar] [CrossRef] [PubMed]
[4]. Franzot SP, Salkin IF, Casadevall A, Cryptococcus neoformans var. grubii: separate varietal status for Cryptococcus neoformans serotype A isolatesJ ClinMicrobiol 1999 37(3):838-40. [Google Scholar]
[5]. Tortorano AM, Viviani MA, Rigoni AL, Cogliati M, Roverselli A, Prevalence of serotype D in Cryptococcus neoformans isolates from HIV positive and HIV negative patients in ItalyMycoses 1997 40(7-8):297-02.10.1111/j.1439-0507.1997.tb00235.x9476513 [Google Scholar] [CrossRef] [PubMed]
[6]. Kauffman CA, Bergman AG, Severance PJ, McClatchey KD, Detection of cryptococcalantigen. Comparison of two latex agglutination testsAm J ClinPathol 1981 75(1):106-09.10.1093/ajcp/75.1.1067457417 [Google Scholar] [CrossRef] [PubMed]
[7]. Day JN, Chau TTH, Wolbers M, Mai PP, Dung NT, Mai NH, Combination antifungal therapy for cryptococcal meningitisN Engl J Med 2013 368(14):1291-02.10.1056/NEJMoa111040423550668 [Google Scholar] [CrossRef] [PubMed]
[8]. Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of americaClin Infect Dis 2010 50:291-322.10.1086/64985820047480 [Google Scholar] [CrossRef] [PubMed]
[9]. Pappas PG, Chetchotisakd P, Larsen RA, Manosuthi W, Morris MI, Anekthananon T, A phase II randomized trial of amphotericin B alone or combined with fluconazole in the treatment of HIV-associated cryptococcal meningitisClin Infect Dis 2009 48(12):1775-83.10.1086/59911219441980 [Google Scholar] [CrossRef] [PubMed]
[10]. Perfect JR, Bennett JE, Dolin R, Blaser MJ, Cryptococcosis (Cryptococcus neoformans and Cryptococcus gattii),”in Principles and Practice of Infectious Diseases 2015 20Elsevier Saunders:2934-48. [Google Scholar]