Introduction
Colorectal carcinoma is a common malignancy with a worldwide distribution. AJCC tumour staging is the single most important prognostic indicator which is also used for selecting cases for postoperative adjuvant therapy. However, a considerable number of curatively treated colon cancer patients ultimately develop disease recurrence prompting the need to identify additional prognostic markers. Extensive research into the biology of colorectal cancer has identified some molecular markers such as Epidermal Growth Factor Receptor (EGFR) which, in addition to providing an insight into the carcinogenesis of colorectal carcinoma, also provide prognostic information.
Aim
To evaluate the prognostic value of expression of EGFR in colorectal carcinoma by correlating it with established prognostic markers such as grade and stage of the tumour.
Materials and Methods
Retrospective cohort study of 100 cases of colorectal carcinoma who underwent radical surgery at a large tertiary care hospital was done. Relevant clinical data of the cases was collected from medical records. Histopathologic evaluation of tumour grade, depth of invasion, number of lymph nodes involved and pathologic stage was done. Immunohistochemistry (IHC) was performed for assessment of EGFR expression, which was categorised as positive if >1% of the tumour cells showed EGFR immune-specific membranous brown staining. Expression of EGFR was correlated with the tumour site, histological grade and the pathologic stage by chi-squared test (χ2). Values were considered significant at p<0.05.
Results
Among the stage I and stage II tumours, 18% and 31%, respectively, were positive for EGFR, while among the stage III and stage IV tumours, 74% and 80%, respectively, were positive for EGFR. A highly positive and significant correlation (p<0.01), along with a linear association was noted between the pathologic stage and the EGFR expression of the tumours. No significant association was noted between either the expression of EGFR and the histological grade (p=0.51) or the expression of EGFR and the site of tumour (p=0.10).
Conclusion
Epidermal growth factor receptor, due to its strong correlation with the pathologic stage of colorectal carcinoma, can be of prognostic significance. This may have a role in selecting those patients who are at high risk for disease progression and therefore are likely to benefit from adjuvant therapy.
Colorectal neoplasms, Molecular markers, Tumour grade
Introduction
Colorectal carcinoma is a common malignancy with a worldwide distribution. Although, it is relatively uncommon in developing countries, the disease has a poorer survival rate with more deaths (52%) in these regions [1]. The single most important prognostic indicator of colorectal carcinoma is the extent of the tumour, or stage at the time of diagnosis [2]. Histological grade is yet another important prognostic indicator [3].
Currently the most widely used system for staging of colorectal carcinoma is the Tumour, Node, Metastasis (TNM) classification and staging system from the American Joint Committee on Cancer (AJCC). Five years survival strongly correlates with the stage of disease at the time of surgical resection [4]. In apparently localised colon cancers, radical surgery with appropriate lymphadenectomy is the mainstay of therapy. The selection of patients who are candidates for postoperative adjuvant medical treatment depends on the AJCC stage arrived at on the resected specimen. Node-negative colon cancer patients are considered to have generally a low risk for systemic disease recurrence and therefore, adjuvant therapy is not indicated in these patients [4,5].
However, a considerable number of curatively treated colon cancer patients ultimately develop disease recurrence. This suggests that AJCC staging may be unable to precisely predict cancer prognosis and that currently available chemotherapy regimens for node-positive patients, although effective, are not completely satisfactory [6,7]. Therefore, a relevant clinical issue is the identification of independent prognostic factors capable of selecting high-risk categories of patients for tailored therapies [6,7].
Studies in the recent years have suggested that the knowledge of molecular events underlying the pathogenesis of colon carcinoma is pivotal in identifying patients with tumours having aggressive biological behaviour [6,7]. Extensive research into the biology of colorectal cancer has identified some molecular markers such as EGFR which, in addition to providing an insight into the carcinogenesis of colorectal carcinoma, also provide prognostic information [7].
Epidermal growth factor receptor is a cell membrane tyrosine kinase receptor that belongs to the ErbB family. When activated by its natural ligands (i.e., epidermal growth factor), EGFR initiates a complex intracellular signal transduction cascade that promotes cancer cell division and migration, angiogenesis and apoptosis inhibition [7,8]. Besides, as the EGFR is a receptor located on the cell surface, it has been found to be a target for molecularly directed therapy [8]. A large number of studies have shown a significant association between high EGFR expression and worse histological grade and advanced AJCC tumour stage at diagnosis. It has also been found to correlate with a more aggressive disease [7,9].
However, other studies have shown some variability of results. Study of McKay JA et al., shows that EGFR expression does not influence patient prognosis [9]. Therefore, the prognostic role of EGFR expression in colon cancer remains a controversial issue.
This study seeks to evaluate the prognostic value of expression of EGFR in colorectal carcinoma by correlating it with established prognostic markers i.e., grade and stage of the tumour.
Materials and Methods
This study was conducted at a large tertiary care center (Base Hospital, New Delhi) in India. The study involved retrospective analysis of cases of colorectal carcinoma that had undergone surgical resection at the center over a period of five years, from November 2011 to October 2016. All cases that had received neoadjuvant therapy were excluded. Formalin fixed, paraffin embedded blocks of primary tumour and regional lymph nodes were retrieved from case files. Relevant clinical data of the cases was collected from medical records.
For histopathologic evaluation of tumour and regional lymph nodes, 4 μ thick sections were obtained from representative blocks of tumour and regional lymph nodes. For each case, at least four sections of the tumour and all sections of regional lymph nodes were obtained. The sections were deparaffinized, rehydrated and stained with routine haematoxylin and eosin (H&E). Slides were critically evaluated for tumour type, grade, depth of invasion, number of lymph nodes involved and pathologic stage. Histologic grade was accorded as per conventional grading of colorectal carcinoma [Table/Fig-1] [10]. Pathologic stage was assessed as per AJCC stage [11].
Histological grade of colorectal carcinoma.
| Grade | Histological features |
|---|
| I | Tumour composed mainly of simple tubulesNuclear polarity discernible; nuclei of uniform size |
| II | Tumour composed mainly of complex, slightly irregular glandsNuclear polarity lost |
| III | >50% of tumour shows solid-like patternNuclear polarity lost |
For IHC, 4 μ thick sections were obtained from representative tumour blocks, deparaffinized and rehydrated. Endogenous peroxidase activity was blocked by immersing in 0.3% hydrogen peroxide for 30 minutes. Antigen retrieval was done by immersing in 0.01 M sodium citrate buffer at pH 6.0 for 40 minutes at 99°C. Mouse monoclonal antibody to EGFR with PharmDx kit (DakoCytomation, Carpenteria, CA) was applied to the tissue sections for 60 minutes at room temperature, followed by biotin labeled goat anti-mouse antibody. Subsequently, biotinylated-avidin coupled horseradish peroxidase was added. On application of diaminobenzidine (Dako), brown complexes were visualised. Counterstaining with haematoxylin was done and the slides were dehydrated and mounted with Permount. For EGFR expression, cases were categorised as positive if >1% of the tumour cells showed EGFR immune-specific membranous brown staining. Positive control for EGFR was colorectal carcinoma strongly positive for EGFR. Negative control consisted of substituting the primary antibody with normal serum.
Statistical Analysis
Expression of EGFR was correlated with the tumour site, histological grade and the pathologic stage by chi-squared test (χ2). Values were considered significant at p<0.05.
Results
The present study comprised 100 cases spanning across a wide range of age. The mean age of the study group was 55.62 years, with the youngest patient being 22 years old, while the oldest, 86 years old. 48% of the subjects were between 40 and 59 years of age, while 38% were between 60 and 79 years of age [Table/Fig-2]. Male:female ratio was 1.3:1.
Age distribution of the study group.
| Age | No. of Cases |
|---|
| 20-29 | 02 |
| 30-39 | 12 |
| 40-49 | 20 |
| 50-59 | 28 |
| 60-69 | 22 |
| 70-79 | 16 |
| Total | 100 |
The most common site of involvement was rectum (40%) followed by sigmoid colon (32%). Tumours of left colon (84%) far outnumbered the tumours of right (10%) and transverse colon (6%) [Table/Fig-3].
Site distribution of the tumours.
| Site | Number of cases | Proportion (%) |
|---|
| Caecum | 04 | 04% |
| Ascending colon | 06 | 06% |
| Transverse colon | 06 | 06% |
| Descending colon | 12 | 12% |
| Sigmoid colon | 32 | 32% |
| Rectum | 40 | 40% |
The study group had adequate representation of all histological grades. Most of the tumours (60%) were grade II [Table/Fig-4]. The Stage distribution of the tumours showed that most of the tumours were of stage III (58%), followed by stage II (26%) and stage I (11%) [Table/Fig-5]. Five patients (5%) had metastatic disease, three of which were found to have peritoneal/liver metastases during elective resection and two underwent emergency surgery for intestinal obstruction.
Grade distribution of the tumours.
| Grade | No. of cases | Percent (%) |
|---|
| I | 27 | 27 |
| II | 60 | 60 |
| III | 13 | 13 |
| Total | 100 | 100 |
Stage distribution of the tumours.
| Stage | No. of cases | Percent (%) |
|---|
| I | 11 | 11 |
| II | 26 | 26 |
| III | 58 | 58 |
| IV | 05 | 05 |
| Total | 100 | 100 |
The expression of EGFR observed in our study is shown in [Table/Fig-6,7]. No significant association were noted between the side of the colon having tumour and expression of EGFR (p=0.19) [Table/Fig-8]. Though, the proportion of EGFR positivity increased with increasing grade of the tumour, no statistically significant correlation could be found (p=0.51) [Table/Fig-9].
Results of immunohistochemistry.
| Expression | EGFR |
|---|
| Positive | 57 |
| Negative | 43 |
| Total | 100 |
EGFR-Epidermal growth factor receptor
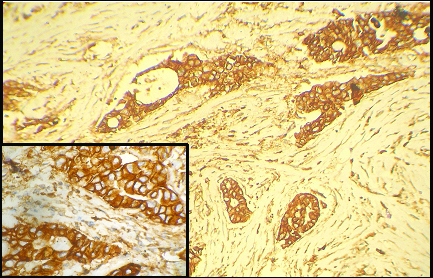
EGFR immunospecific tumour cells. (x100); Inset: (x400).
Correlation of expression of EGFR with right and left colonic tumours.
| Side of colon | EGFR Positive | EGFR Negative | Total |
|---|
| Right | 04 | 06 | 10 |
| Left | 50 | 34 | 84 |
EGFR-Epidermal growth factor receptor
Correlation of expression of EGFR with tumour grade.
| Grade | EGFR Positive | EGFR Negative | Total |
|---|
| I | 12 (44%) | 15 (55%) | 27 (100%) |
| II | 35 (58%) | 25 (41%) | 60 (100%) |
| III | 10 (76%) | 03 (23%) | 13 (100%) |
EGFR-Epidermal growth factor receptor
Among the stage I and stage II tumours, only 18% and 31%, respectively, were positive for EGFR, while among the stage III tumours and the stage IV tumours, 74% and 80%, respectively were positive for EGFR [Table/Fig-10]. There was a highly positive and significant correlation (p<0.01), along with a linear association, between the stage and the EGFR expression of the tumours.
Correlation of expression of EGFR with tumour stage.
| Stage | EGFR Positive | EGFR Negative | Total |
|---|
| I | 02 (18%) | 09 (82%) | 11 (100%) |
| II | 08 (31%) | 18 (69%) | 26 (100%) |
| III | 43 (74%) | 15 (26%) | 58 (100%) |
| IV | 04 (80%) | 01 (20%) | 05 (100%) |
| Total | 57 | 43 | 100 |
EGFR-Epidermal growth factor receptor
Discussion
The incidence of colorectal carcinoma increases with age. Western literature reports a peak age of incidence between 60 and 70 years with fewer than 20% of cases occurring before the age of 50 [2]. However, in our study group, the maximum proportion of cases (28%) were a decade younger and one third of the cases occurred before 50 years of age. Mohandas KM and Desai DC, too have noted the occurrence of rectal cancer in a younger age group in India [12]. The male to female ratio, in present study, was 1.3:1; this is comparable with the ratio of 1.3:1 to 1.5:1 reported in literature [1,12].
The most common site of involvement was rectum (40%). Tumours of left colon (84%) far outnumbered the tumours of right and transverse colon (16%). Other studies, too, have noted a higher incidence of tumours of the left side as compared to the right. Mohandas KM and Desai DC found in their study that 77% of the tumours occurred in the left colon [12].
The stage distribution of the tumours showed that most of the tumours were of stage III (58%). Only 5% of the tumours were of stage IV. Another study of different ethnic populations has noted that 35-40% of the tumours at the time of diagnosis were in stages I and II, 32-36% were in stage III and 19-24% were in stage IV [13]. A smaller proportion of stage IV cases in present study may be accounted for by the fact that present study group included only operated cases and surgery is not the mainstay of treatment for stage IV disease with widespread metastasis.
Present study has found expression of EGFR in 57% of tumours. Expression of EGFR in colorectal carcinoma has been demonstrated by various studies to be between 53% and 97% with a great variability of results. This may be due to different methods of detecting EGFR protein expression and different cut-off values used for defining expression [7,14,15].
Present study showed no differences in EGFR expression among tumours of different sides of the colorectal tract. This finding is consistent with other published articles [7,14].
Among the stage I and stage II tumours, only 18% and 31%, respectively were positive for EGFR, while among stage III and stage IV tumours, 74% and 80%, respectively were positive for EGFR. There was a highly positive and significant correlation (p<0.01), along with a linear association, between the stage and EGFR expression of the tumours. Other studies, too have highlighted a similar relationship between EGFR expression and tumour stage [7,16].
This study has also attempted to compare the expression of EGFR protein with tumour grade. No association between the two was noted (p=0.51). Other studies have shown controversial results on the subject, with some studies showing a link and others reporting no link between histological grade and EGFR levels [7,9,16,17].
Limitation
The limitation of present study is that it has comprised only operated cases, thereby including a relatively small number of stage IV cases. This may not be a true reflection of EGFR expression among stage IV tumours. Not withstanding this, EGFR expression has shown a significant positive correlation with tumours of a higher stage.
A substantial subset of colon cancer patients undergoing potentially curative surgery develop recurrence [6,7]. Hence, the knowledge of molecular features underlying the behaviour of individual colon tumours is a fundamental step in identifying patients with a high risk of recurrence.
Conclusion
Epidermal growth factor receptor, by its strong association with the TNM stage of colorectal carcinoma, can be of prognostic significance. This may have a role in selecting those patients who are at high risk for disease progression and therefore are likely to benefit from adjuvant therapy.
EGFR-Epidermal growth factor receptor
EGFR-Epidermal growth factor receptor
EGFR-Epidermal growth factor receptor
EGFR-Epidermal growth factor receptor
[1]. GLOBOCAN. Estimated cancer incidence, mortality and prevalence worldwide in 2012. 2012. http://globocan.iarc.fr [Google Scholar]
[2]. Liu C, Crawford JM, The Gastro-intestinal Tract. In: Kumar V, Abbas AK, Fausto N, Aster J, editorsRobbins and cotran pathologic basis of disease 2010 8th edPhiladelphiaSaunders:1464 [Google Scholar]
[3]. Ueno H, Mochizuki H, Hashiguchi Y, Ishiguro M, Kajiwara Y, Sato T, Histological grading of colorectal cancer: a simple and objective methodAnn Surg 2008 247(5):811-18.10.1097/SLA.0b013e318167580f18438118 [Google Scholar] [CrossRef] [PubMed]
[4]. Zaniboni A, Labianca R, Adjuvant therapy for stage II colon: an elephant in the living roomAnn Oncol 2004 15(9):1310-18.10.1093/annonc/mdh34215319235 [Google Scholar] [CrossRef] [PubMed]
[5]. Sobrero A, Guglielmi A, Current controversies in the adjuvant therapy of colon cancerAnn Oncol 2004 15(Suppl 4):39-41.10.1093/annonc/mdh90315477333 [Google Scholar] [CrossRef] [PubMed]
[6]. Galizia G, Lieto E, Ferrarraccio F, Orditura M, De Vita F, Castellano P, Determination of molecular marker expression can predict clinical outcome in colon carcinomasClin Cancer Res 2004 10(10):3490-9.10.1158/1078-0432.CCR-0960-0315161706 [Google Scholar] [CrossRef] [PubMed]
[7]. Spano JP, Lagorce C, Atlan D, Milano G, Domont J, Benamouzig R, Impact of EGFR expression on colorectal cancer patient prognosis and survivalAnn Oncol 2005 16(1):102-08.10.1093/annonc/mdi00615598946 [Google Scholar] [CrossRef] [PubMed]
[8]. Grunwald V, Hidalgo M, Developing inhibitors of the epidermal growth factor receptor for cancer treatmentJ Natl Cancer Inst 2003 95(12):851-67.10.1093/jnci/95.12.85112813169 [Google Scholar] [CrossRef] [PubMed]
[9]. McKay JA, Murray LJ, Curran S, Ross VG, Clark C, Murray GI, Evaluation of the epidermal growth factor receptor (EGFR) in colorectal tumours and lymph node metastasesEur J Cancer 2002 38(17):2258-64.10.1016/S0959-8049(02)00234-4 [Google Scholar] [CrossRef]
[10]. Cooper HS, Alimentary canal and associated organs. In: Mills SE, Carter D, Greenson JK, editorsSternberg’s diagnostic surgical pathology 2004 4th edPhiladelphiaLippincott Williams and Wilkins [Google Scholar]
[11]. AJCC Cancer Staging Manual, 7th ed. Published by Springer, New York. 2010. www.springeronline.com [Google Scholar]
[12]. Mohandas KM, Desai DC, Epidemiology of large and small bowel cancers in IndiaIndian J Gastroenterol 1999 18(3):118-21. [Google Scholar]
[13]. American Cancer Society. Colorectal cancer facts & figures 2017-2019. Atlanta: American Cancer Society. 2017;01-36 [Google Scholar]
[14]. Goldstein NS, Armin M, Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint Committee on Cancer stage IV colon adenocarcinoma: implications for a standardized scoring systemCancer 2001 92(5):1331-46.10.1002/1097-0142(20010901)92:5<1331::AID-CNCR1455>3.0.CO;2-M [Google Scholar] [CrossRef]
[15]. Scartozzi M, Bearzi I, Berardi R, Mandoles A, Frabris G, Cascinu S, Epidermal growth factor receptor (EGFR) status in primary colorectal tumors does not correlate with EGFR expression in related metastatic sites: implications for treatment with EGFR-targeted monoclonal antibodiesJ Clin Oncol 2004 22(23):4772-78.10.1200/JCO.2004.00.11715570078 [Google Scholar] [CrossRef] [PubMed]
[16]. Karameris A, Kanavaros P, Aninos D, Gorgoulis V, Mikou G, Rokas T, Expression of epidermal growth factor (EGF) and epidermal growth factor receptor (EGFR) in gastric and colorectal carcinomas. An immunohistological study of 63 casesPathol Res Pract 1993 189(2):133-37.10.1016/S0344-0338(11)80082-8 [Google Scholar] [CrossRef]
[17]. Steele RJ, Kelly P, Ellul B, Eremin O, Immunohistochemical detection of epidermal growth factor receptors on human colonic carcinomasBr J Cancer 1990 61(2):325-26.10.1038/bjc.1990.632178668 [Google Scholar] [CrossRef] [PubMed]