Spindle Cell Lipoma of the Arm: A Case Report with Review of Literature
Surya Rao Rao Venkata Mahipathy1, Alagar Raja Durairaj2, Narayanamurthy Sundaramurthy3, Senthil Kumar Azhisoor Chandrasekhar4, Jayaganesh Parthasarathy5
1 Associate Professor, Department of Plastic and Reconstructive Surgery, Saveetha Medical College and Hospital, Chennai, Tamil Nadu, India.
2 Associate Professor, Department of Plastic and Reconstructive Surgery, Saveetha Medical College and Hospital, Chennai, Tamil Nadu, India.
3 Assistant Professor, Department of Plastic and Reconstructive Surgery, Saveetha Medical College and Hospital, Chennai, Tamil Nadu, India.
4 Assistant Professor, Department of Surgical Oncology, Saveetha Medical College and Hospital, Chennai, Tamil Nadu, India.
5 Associate Professor, Department of Pathology, Saveetha Medical College and Hospital, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Surya Rao Rao Venkata Mahipathy, Associate Professor, Department of Plastic and Reconstructive Surgery, Saveetha Medical College and Hospital, Thandalam, Kanchipuram-602105, Chennai, Tamil Nadu, India.
E-mail: surya_3@hotmail.com
Spindle cell lipomas are rare benign tumours of adipocytes. They are usually found in middle aged to elderly males. There are a number of differential diagnoses for this lesion. Treatment is excision with no recurrence. Here we report a case of a firm non tender swelling of the arm which after excision was diagnosed as Spindle Cell Lipoma (SCL). We discuss the case and a review of the literature for a deeper understanding of this tumour.
Benign, Excision, Lipomatous
Case Report
A 65-year-old female presented with a large mass over the left lower arm that had been present since one month. She was apparently normal till one month ago when she noticed a small swelling on the left upper arm which was insidious in onset and gradually progressed to the present size. She complained of dull pain over the swelling, but no history of trauma, fever, bleeding or ulceration of the swelling. On examination, there was a 5×4 cm swelling over the flexor aspect of left arm just above the cubital fossa to 5 cm above. It was oval in shape with illdefined borders and an irregular surface. On palpation, the swelling was firm in consistency with a 1×1 cm cystic nodule at the summit of the swelling. It was in a subcutaneous plane with the overlying skin free. Mobility was restricted on contracting the flexor muscles of the left arm. There was no regional lymphadenopathy. A clinical diagnosis of a soft tissue sarcoma was made [Table/Fig-1].
Preoperative photograph of the lesion.

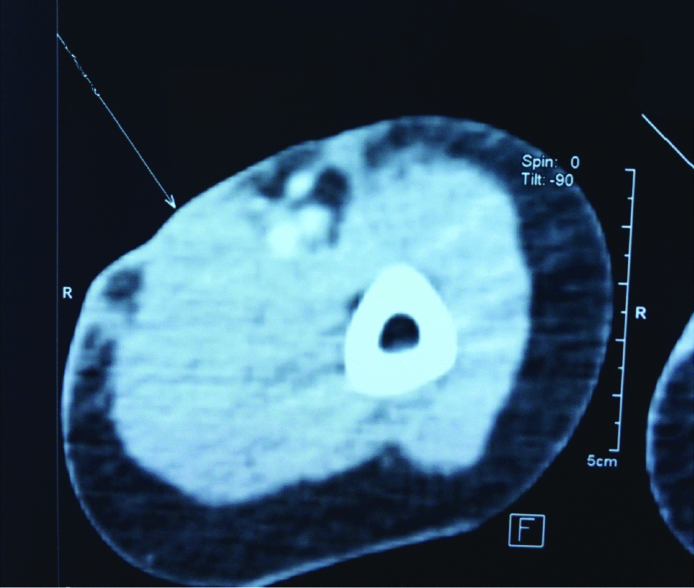
Ultrasound revealed an illdefined echogenic lobulated soft tissue thickening noted in the cutis and subcutis planes of the volar aspect of left distal arm measuring 1.7 cm in its maximum depth. No sonographically detectable muscular plane extension noted with lesion ending just proximal to cubital fossa and no evidence of collections. Patient was not cooperative for MRI and hence Computed Tomography (CT) was taken. CT showed a focal thickening measuring 5.7×3.7×0.6 cm with no significant enhancement seen in skin and subcutaneous plane in flexor compartment of left mid and distal arm. There was no obvious invasion of the lesion into the flexor compartment of left arm. Adjacent brachial artery and vein shows normal calibre and enhancement with no evidence of invasion [Table/Fig-2]. A provisional diagnosis of a soft tissue tumour was given.
CT showing a focal thickening measuring (5.7x3.7x0.6) cm with no significant enhancement seen in skin and subcutaneous plane in flexor compartment of left distal arm with no invasion into the flexor compartment of left arm and normal brachial vasculature.
Tru-cut biopsy was done which showed linear fragments of tissue with numerous skeletal muscle fibres with scattered areas showing spindle cells with mildly pleiomorphic nuclei seen in sheets and in haphazard pattern in a myxoid stroma. No evidence of necrosis was noted. Features were suggestive of low grade spindle cell lesion.
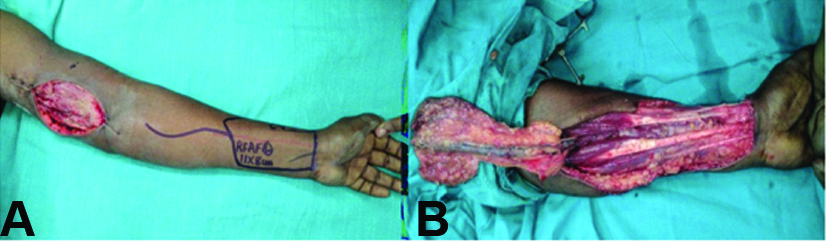
After informed consent was obtained, the patient was taken to the operating room for excisional biopsy. An elliptical incision was made overlying the mass. The mass was then dissected circumferentially and removed with a cuff of flexor muscle along with the overlying skin, with a 2 cm 3D clearance. The specimen was sent for histopathology. The defect was 11×8 cm and was covered with a reverse radial artery forearm flap [Table/Fig-3,4a,b] after doing an Allen’s test and Hand-held Doppler study, and the donor area was resurfaced with a split skin graft [Table/Fig-5].

a) Reverse radial artery forearm flap planned and marked; b) Flap raised.
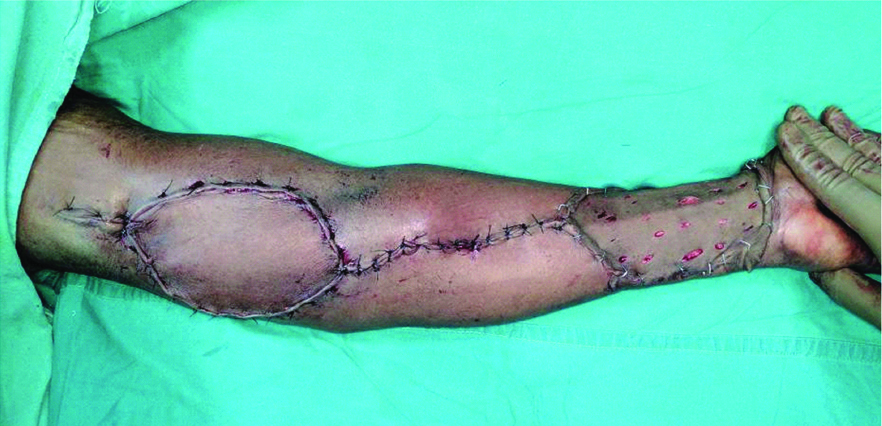
Immediate postoperative picture.
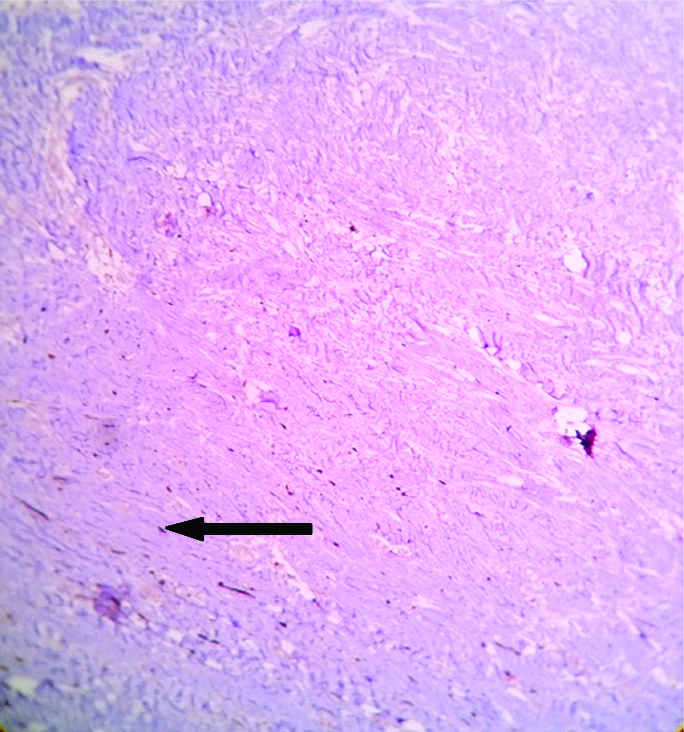
On gross examination, skin covered soft tissue measuring 8×6×2.5 cm was present with cut surface showing yellowish and grey white areas with no definite tumour made out. Microscopy showed epidermis with adnexal structures and a lesion beneath that was composed of lobules of adipocytes and sheets and haphazard arrangement of bland spindle cells. There were areas showing cells around neural bundles, and in an angiomatous vascular pattern there were scattered ropy thick collagen bundles and focal lobules of adipocytes showing cells with cytoplasm having many small vacuoles and a central placed nucleus [Table/Fig-6a,b]. The margins marked are free of tumour. The spindle cells were negative for S-100 protein [Table/Fig-7]. The features were suggestive of SCL.
Microscopy showing lobules of; a) adipocytes (red arrow) with; b) intervening fibrous septa (green arrow) (40X).
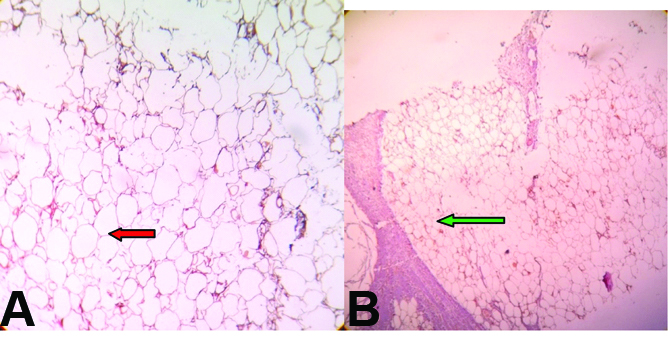
Microscopy shows spindle cells (black arrow) in sheets negative for S100 protein (40X).
Post procedure, the flap was well settled and the donor graft uptake was complete [Table/Fig-8]. Since, the diagnosis was SCL, no adjuvant treatment was required. The patient was on regular follow up for one year with no recurrence.
Two months postoperative picture.

Discussion
Spindle cell lipomas are relatively rare, slow growing, benign lipomatous tumours. SCL is a distinct histological variant of lipoma derived from prelipoblastic mesenchymal cells [1]. These masses usually occur in males around the 4th to 7th decade of life and originate most frequently in the subcutaneous tissue of the upper back, posterior neck, and shoulders [2-4]. SCLs account for only 1.5% of all reported lipomatous tumours and these lesions share similar morphology with other fatty/spindle cell or myxoid lesions, benign and malignant [4-5]. Prognosis is excellent and thus, wide local excision is the treatment of choice [2,6].
Lipomatous tumours like lipomas arise from mesenchymal tissue [7]. The probable source of origin of SCLs are fibroblasts and preadiposites [8].
Spindle cell lipomas with unusual histological growth pattern and cytology can be confused with liposarcomas, other spindle cell tumours and myxoid lesion [2,4,5,7-9]. Liposarcomas are usually located in a deeper plane [10].
Cytology, light and electron microscopy, and cytogenetic studies are tools used to aid in the differentiation of benign and malignant lipomatous tumours. As in our case, SCLs are composed of three basic histologic components being mature adipocytes, uniform spindle cells, and bundles of collagen with a prominent myxoid matrix which is occasionally seen [2,4,8]. Mitotic figures are scarce [8]. A portion of diagnostic difficulty lies in the fact that SCL may show considerable variation in the proportion of each component, yet the great majorities have significant amounts of mature adipocytes and spindle cells [4,9]. Other tumours with similar morphology are increasingly described as are SCLs of unusual sites, thus making correct differentiation vital.
Spindle cell lipomas can be differentiated from liposarcomas by CD34 positivity and absence of lipoblast cells in SCLs [2,10]. They also have characteristic karyotypic aberrations like loss of material from long arms of chromosomes 13 or 16.
Magnetic resonance imaging is primarily useful in assessing the extent of the lesion as it may be difficult to differentiate from liposarcomas. Fine needle aspiration shows adipocytes, spindle cells and collagen fibres. But the proportion of these elements may vary and made it difficult to differentiate from myxoid lesions [4]. Hence, excisional biopsy is recommended.
Two studies that followed patients for up to 22 and 25 years following surgical excision of the spindle cell lipoma reported a benign clinical course and proved that treatment with local excision was curative [2,6].
Rarely, SCLs can be multiple when heriditary and also be found in atypical locations like scalp, orbit, buccal mucosa, breast and subungual tissues [5,11-15].
Conclusion
Spindle cell lipomas are rare, benign lipomatous tumours usually occuring in the posterior neck, upper back, and shoulder region in males aged 40-70 years. A number of cytogenetic tests are available as an adjunct to clinical evaluation and hisopathological examination to support the diagnosis. Surgical excision of SCL is considered curative.
[1]. Piattelli A, Perrotti V, Fioroni M, Rubini C, Spindle cell lipoma of the floor of the mouth: report of a caseAuris Nasus Larynx 2005 32(2):205-07. [Google Scholar]
[2]. Enzinger FM, Harvey DA, Spindle cell lipomaCancer 1975 36(5):1852-59. [Google Scholar]
[3]. Chandrashekhar P, Jose M, Dadhich M, Chatra L, Holla V, Spindle cell lipoma: a case report and review of literatureKathmandu Univ Med J 2012 10(38):92-95. [Google Scholar]
[4]. Domanski HA, Carlén B, Jonsson K, Mertens F, Akerman M, Distinct cytologic features of spindle cell lipoma. A cytologic-histologic study with clinical, radiologic, electron microscopic, and cytogenetic correlationsCancer 2001 93(6):381-89. [Google Scholar]
[5]. Thompson LD, Spindle-cell lipomaEar Nose Throat J 2009 88(7):992-93. [Google Scholar]
[6]. Angervall L, Dahl I, Kindblom LG, Spindle cell lipomaActa Pathol Microbiol Scand A 1976 84(6):477-87. [Google Scholar]
[7]. Weiss SW, Lipomatous tumorsMonogr Pathol 1996 38:207-39. [Google Scholar]
[8]. Bolen JW, Thorning D, Spindle-cell lipoma. A clinical, light-and electron-microscopical studyAm J Surg Pathol 1981 5(5):435-41. [Google Scholar]
[9]. Billings SD, Folpe AL, Diagnostically challenging spindle cell lipomas: a report of 34 “low-fat” and “fat-free” variantsAm J Dermatopathol 2007 29(5):437-42. [Google Scholar]
[10]. Comunoglu N, Comunoglu C, Ekici AI, Ozkan F, Dervisoglu S, Spindle cell lipomaPol J Pathol 2007 58(1):07-11. [Google Scholar]
[11]. Fanburg-Smith JC, Devaney KO, Miettinen M, Weiss SW, Multiple spindle cell lipomas: a report of 7 familial and 11 nonfamilial casesAm J Surg Pathol 1998 22(1):40-48. [Google Scholar]
[12]. Ulivieri S, Olivieri G, Motolese PA, Frushelli M, Motolese E, Menicacci F, Spindle cell lipoma of the orbit: a case report of an unusual orbital pathologyNeurol Neurochir Pol 2010 44(4):419-23. [Google Scholar]
[13]. Kwon NH, Kim HS, Kang H, Lee JY, Kim HO, Kang SJ, Subungual spindle cell lipomaInt J Dermatol 2013 52(5):636-38. [Google Scholar]
[14]. Mulvany NJ, Silvester AC, Collins JP, Spindle cell lipoma of the breastPathology 1999 31(3):288-91. [Google Scholar]
[15]. Haas AF, Fromer ES, Bricca GM, Spindle cell lipoma of the scalp: a case report and reviewDermatol Surg 1999 25(1):68-71. [Google Scholar]