Pancytopenia is a diagnostically challenging entity encountered commonly in routine clinical practice. It is not a disease entity by itself but an expression of an underlying disorder that can either be a haematopoietic or a non haematopoietic condition. It is characterised by the reduction of Red Blood Cells (RBCs), White Blood Cells (WBCs) and platelets below the normal reference range [1]. Initially, most of the clinical manifestations can be attributed to anaemia and thrombocytopenia, however, leukopenia is encountered late in the disease [2]. The underlying mechanisms maybe secondary to decreased marrow production, marrow infiltration, bone marrow suppression, ineffective haematopoiesis or increased peripheral destruction [2,3]. There are many studies in the literature stating the various causes of pancytopenia [1-3]. This study aimed to evaluating the clinicopathological profile of pancytopenia. The study also aimed to know whether there is a need for a bone marrow examination in every case of pancytopenia and also the importance of ancillary tests to identify the cause. To the best of our knowledge, this is the largest study from the Indian subcontinent.
Materials and Methods
The present descriptive study was performed in the haematology laboratory of a Kasturba Medical College, Mangaluru, India (from January 2010 to December 2016). Cases with evidence of pancytopenia having Haemoglobin (Hb) less than 11 gm/dL, TLC less than 4000 cells/mm3, Absolute Neutrophil Count (ANC) of less than 1500 cells/mm3 and PC less than 150000/mm3 formed the core of the study [4]. Patients of all age groups were included in the study while pancytopenia secondary to myelotoxic chemotherapy were excluded. A detailed relevant history with physical examination findings were evaluated. Samples collected in ethylenediaminetetraacetic acid anticoagulated vacutainer were processed through automated haematology analyser (SYSMEX XT-1800i). Laboratory parameters that were analysed included Hb, RBC count, TLC, Differential Leukocyte Count (DLC), ANC and PC. Red cell indices that included Mean Corpuscular Volume (MCV), Mean Corpuscular Haemoglobin (MCH), Mean Corpuscular Haemoglobin Concentration (MCHC), PCV and Red Cell Distribution Width (RDW) were also analysed along with reticulocyte count and Reticulocyte Production Index (RPI). Peripheral smear, stained using Leishman’s stain, was evaluated. Bone marrow aspiration was performed only in those cases which had an absolute indication for pancytopenia and the slides were reviewed. Informed consent was taken from the patients due for bone marrow aspiration. Ethical clearance was obtained from the Institutional Ethics Committee.
Statistical Analysis
Data was analysed using SPSS 22.0 version software and the test of significance was performed by chi-square test. A p-value <0.05 was considered of statistical significant.
Results
A total of 400 patients with pancytopenia were included in the study, and bone marrow aspiration was performed in 200 cases, out of which biopsy correlation was available in 77 cases. The age ranged from two days to 90 years (mean±SD age: 34±19.6 years). The maximum number of cases was noted in fourth decade. There were 245 males and 155 females with male to female ratio of 1.6:1. In the present study, 82 cases were seen in paediatric age group (2 days- 18 years, mean age=8 years).
The most common presenting complaint was weakness and easy fatigability (n=337, 84.3%) and pallor was the most common physical sign (n=400, 100%). The clinical features and findings of physical examination are tabulated and distribution of various causes of pancytopenia [Table/Fig-1,2].
Clinical findings in pancytopenia.
| Presenting symptoms and signs | Number of cases | Percentage of cases (%) |
|---|
| Presenting symptoms |
| Weakness and easy fatigability | 337 | 84.3% |
| Fever | 251 | 62.8% |
| Chills and rigors | 147 | 36.75% |
| Nausea and vomiting | 125 | 31.25% |
| Loss of weight | 120 | 30% |
| Exertional breathlessness | 89 | 22.25 |
| Cough and expectoration | 25 | 6.25% |
| Bleeding manifestations | 72 | 18% |
| Sternal tenderness | 19 | 4.75% |
| Myalgia | 12 | 3% |
| Physical signs |
| Pallor | 400 | 100% |
| Splenomegaly | 267 | 66.75% |
| Haepatomegaly | 200 | 50% |
| Bald tongue | 73 | 18.25% |
| Bleeding signs | 72 | 18% |
| Icterus | 53 | 13.25% |
| Angular stomatitis | 53 | 13.25% |
| Oedema | 48 | 12% |
| Lymphadenopathy | 48 | 12% |
| Gum hypertrophy | 7 | 1.7% |
Distribution of various causes of pancytopenia.
| Diseases | Number of cases | Percentage of cases (%) | M:F Ratio | Age range |
|---|
| Infections | 175 | 43.8% | 1.8:1 | 7-68 years |
| Malaria | 108 | 27% | 1.7:1 | 7-47 years |
| Plasmodium vivax | 52 | 13% | 1.3:1 | 7-66 years |
| Mixed vivax and falciparum | 41 | 10.25% | 1.2:1 | 15-68 years |
| Plasmodium falciparum | 15 | 3.75% | 1.3:1 | 10-52 years |
| HIV | 33 | 8.25% | 2.3:1 | 8-61 years |
| Sepsis | 19 | 4.75% | 0.6:1 | 1 day-44 years |
| Dengue fever | 08 | 2% | 1.7:1 | 8-50 years |
| Tuberculosis | 05 | 1.25% | 4:1 | 39-68 years |
| Viral fever | 02 | 0.5% | 1:1 | 29-33 years |
| Nutritional |
| Megaloblastic anaemia | 93 | 23.25% | 1.8:1 | 17-87 years |
| Neoplastic conditions | 50 | 12.5% | 1.1:1 | 2-81 years |
| Acute myeloid leukaemia | 13 | 3.25% | 0.7:1 | 2-81 years |
| AML-M2 | 06 | 1.5% | 2.5:1 | 4-81 years |
| AML-M4 | 04 | 1% | 0.6:1 | 16-30 years |
| APL | 03 | 0.75% | 1:2 | 2-24 years |
| Acute lymphoid leukaemia | 12 | 3% | 0.8:1 | 2-77 years |
| Hodgkin’s lymphoma | 01 | 0.25% | Male | 65 years |
| Non-Hodgkin’s lymphoma | 07 | 1.75% | 2.5:1 | 34-69 years |
| DLBCL | 04 | 1% | 3:1 | 34-64 years |
| Follicular lymphoma | 01 | 0.25% | Female | 55 years |
| Peripheral T-cell lymphoma | 01 | 0.25% | Male | 69 years |
| Atypical burkitt lymphoma | 01 | 0.25% | Male | 52 years |
| MDS | 08 | 2% | 1:1 | 45-80 years |
| Myelofibrosis | 07 | 1.75% | 1.7:1 | 29-68 years |
| Multiple myeloma | 02 | 0.5% | Male | 72 years |
| Hypoplastic marrow | 23 | 5.75% | 0.6:1 | 2-80 years |
| Idiopathic aplastic anaemia | 11 | 2.75% | 2:1 | 22-80 years |
| Fanconi anaemia | 08 | 2% | 1.3:1 | 2-14 years |
| Drug induced aplastic anaemia | 03 | 0.75% | 2:1 | 18-35 years |
| Hepatitis B infection induced | 01 | 0.25% | Female | 22 years |
| Chronic Liver Disease (CLD) | 57 | 14.25% | 4.2:1 | 13-90 years |
| Alcoholic liver disease | 36 | 9% | 17:1 | 24-76 years |
| Viral haepatitis | 13 | 3.25% | 1.6:1 | 13-90 years |
| Autoimmune hepatitis | 04 | 1% | 1:3 | 25-42 years |
| Nonalcoholic liver disease | 03 | 0.75% | 2:1 | 16-76 years |
| Mucolipidosis | 01 | 0.25% | Male | 4 months |
| Primary hypersplenism | 02 | 0.5% | 1:1 | 45-55 years |
M- Male, F-Female, AML-M2- Acute myeloblastic leukaemia with maturation, AML-M4- Acute myelomonocytic leukaemia, APL- Acute promyelocytic leukaemia, DLBCL- Diffuse large B-cell lymphoma, MDS- Myelodysplastic syndrome, HIV- Human immunodeficiency virus
Pancytopenia in Infections
Infections was the most common disease category in the present study which presented with pancytopenia and malaria was the most common infection (n=108, 27%) cases. Plasmodium vivax was observed in 52 cases, mixed infection of Plasmodium vivax and Plasmodium falciparum in 41 cases and remaining 15 cases were of Plasmodium falciparum infection [Table/Fig-3a]. The diagnosis of malaria was established by peripheral smear examination and only one case required bone marrow evaluation as it was referred as pancytopenia in a case of pyrexia of unknown origin. On follow up, all patients had haematological recovery with normalisation of blood counts after anti-malarial treatment. Pancytopenia was a common presentation in retroviral disease patients with 23 males and 10 females. Seventeen patients of neonatal sepsis presented as pancytopenia, similarly 30-year-old postpartum patient and a 44-year-old male with multi organ dysfunction syndrome also presented with pancytopenia secondary to sepsis.
a) Pancytopenia in malaria: a case of pyrexia of unknown origin with gametocyte of Plasmodium falciparum (arrow) detected in the marrow along with few myeloid precursors (arrow head) (Leishman; 200X). Inset: Bone marrow showing numerous malaria pigment laden macrophages (Leishman; 400X); b) Pancytopenia in tuberculosis: a case of persistent pancytopenia evaluated with bone marrow showing epitheliod granulomas (Leishman; 100X). Inset-left: Epithelioid granuloma with Langhan’s giant cell (H&E, 200X). Inset-right: Positive acid fast bacilli in marrow aspirate (Ziehl Neelson; 1000X); c,d) Pancytopenia in disseminated histoplasmosis in a HIV case; c) Disseminated cutaneous nodules with conjunctival involvement. Inset-left: Bone marrow aspirate showed macrophages with engulfed histoplasmosis (Leishman; 1000X). Inset-right: Largest cutaneous nodule on the left nasal ala; d) The conjunctival biopsy showed granulomatous infiltrate and histiocytes with intracellular histoplasma spores (H&E, 1000X). Inset: Intracellular histoplasma capsulatum (H&E, 1000X).
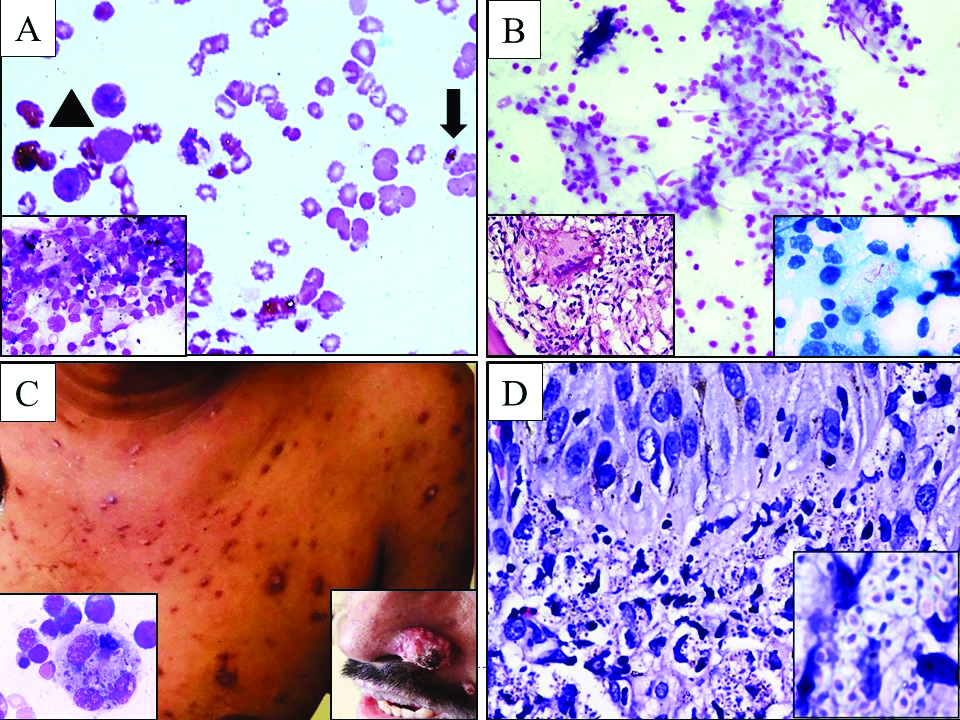
We encountered five cases of tuberculosis which showed the presence of epithelioid granulomas and Acid-Fast Bacilli (AFB) positivity in bone marrow [Table/Fig-3b]. The blood counts improved after Anti-Tubercular Therapy (ATT) treatment in these patients. Transient pancytopenia was evidenced in patient with dengue fever (n=8, 2%) and viral fever (n=2, 0.5%).
One case of retroviral disease with disseminated histoplasmosis was encountered. The bone marrow showed numerous macrophages with intracytoplasmic histoplasma capsulatum organisms. The Periodic Acid-Schiff (PAS) stain demonstrated the capsule of the organism [Table/Fig-3c,d].
Pancytopenia in Anaemia
Megaloblastic anaemia (n=93, 23.25%) was the second most common disease category in the present study. Morphological evidence of associated microcytic hypochromic anaemia was observed in (n=65, 69.9%). These cases were evaluated for serum vitamin B12 and folic acid assays. The aetiology observed in the study group were vitamin B12 deficiency (n=27, 29%), folic acid deficiency (n=12, 12.9%), combined vitamin B12 and folic acid deficiency (n=6, 6.5%), chronic liver disease (n=4, 4.5%) and malabsorption syndrome (n=1, 0.25%).
Pancytopenia with Malignancy
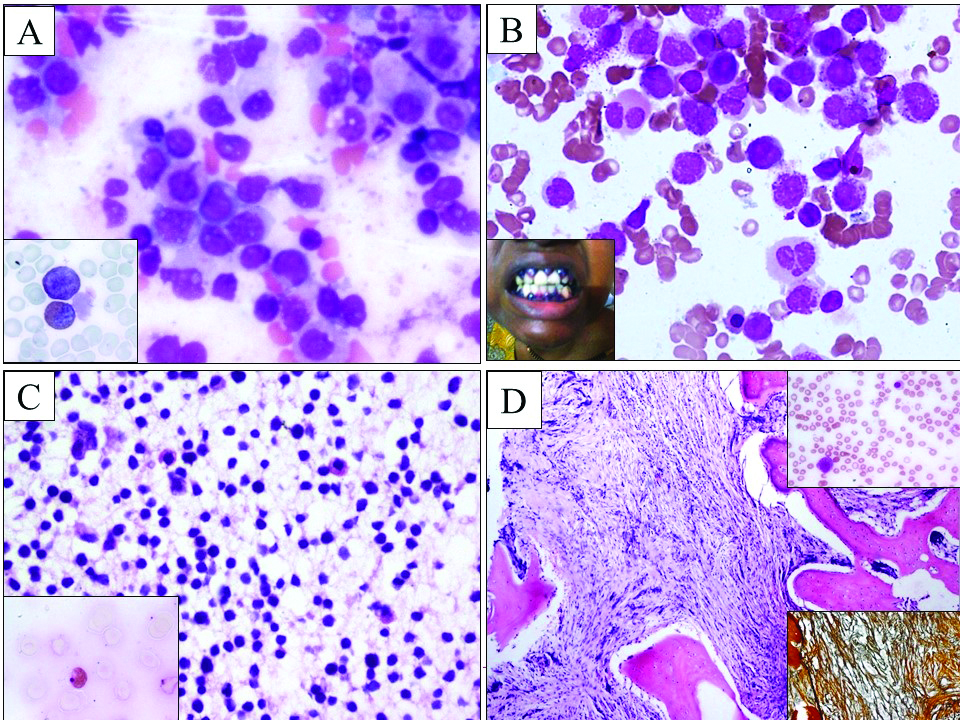
In the present study, a wide range of neoplastic conditions presented with pancytopenia as depicted and [Table/Fig-2,4a-d]. Four cases of Acute Myelomonocytic Leukaemia (AML-M4) had developed in a background of myelodysplastic syndrome. Atypical Burkitt’s lymphoma patient was a 52-year-old man with fever, cough, expectoration and unexplained bleeding tendency. A terminal ileal ulcer biopsy revealed extranodal atypical Burkitt’s lymphoma. The neoplastic cells were CD45, CD20, Ki-67 and CD10 positive with focal positivity to BCL2 and were MUM1 negative. Bone marrow involvement was seen. Hodgkin’s lymphoma case showed involvement of bone marrow with lymph node biopsy revealing classical Hodgkin’s lymphoma (lymphocyte depletion type; diffuse fibrosis subtype). On immunohistochemistry, the Reed-sternberg cells were CD15 and CD30 positive, and CD20 negative.
a) Pancytopenia in AML: bone marrow aspirate showing myeloblasts (400X; Leishman). Inset: MPO positivity in blasts (1000X; Leishman); b) Pancytopenia in AML: bone marrow aspirate with abnormal hypergranular promyelocytes in a case of APL (400X; Leishman). Inset: a case of AML-M4 with gum hypertrophy; c) Pancytopenia in all: bone marrow biopsy with diffuse infiltration by lymphoblasts.(200X; H&E). Inset: PAS positivity in lymphoblasts (1000X; Leishman); d) Pancytopenia in myelofibrosis: bone marrow biopsy showing extensive fibrosis (40X; H&E). Inset-top right: Leucoerythroblastic blood picture in myelofibrosis (400X; Reticulin stain). Inset-bottom right: Grade 3 reticulin fibres in marrow (40X; Reticulin stain).
Pancytopenia with Hypoplastic Marrow
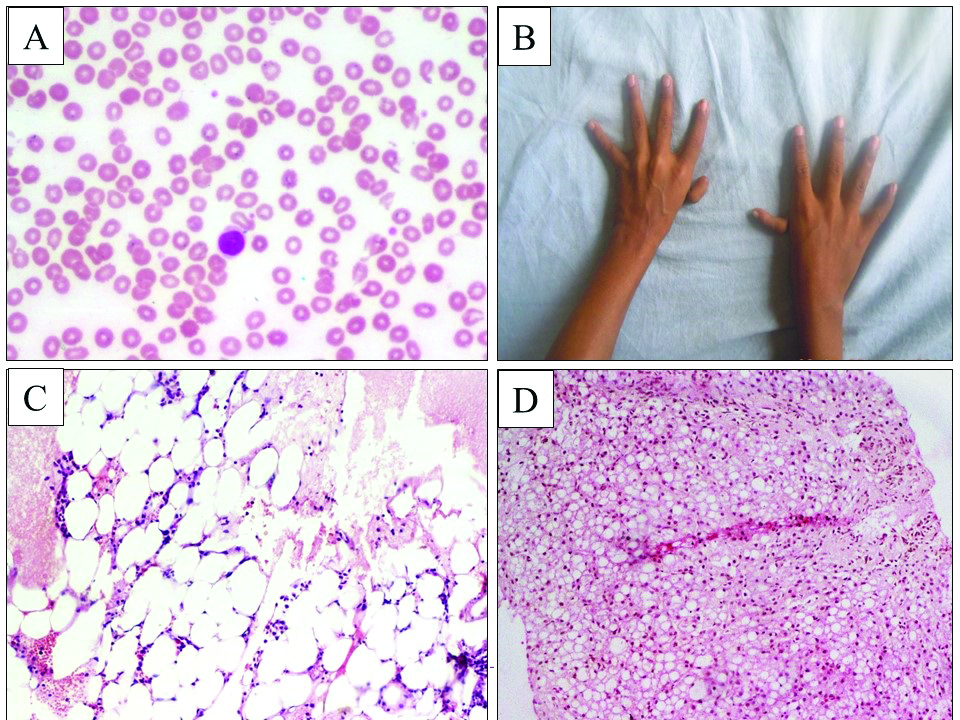
Pancytopenia secondary to hypoplastic marrow was seen in 23 (5.75%) patients in present study. On evaluation, these cases were categorised as idiopathic aplastic anaemia (n=11, 2.75%), Fanconi anaemia (n=8, 2%), drug induced hypoplastic marrow (n=3, 0.75%) and hepatitis B (n=1, 0.25%) [Table/Fig-5a-c]. Patient with hepatitis B infection case was referred for evaluation of severe pancytopenia where marrow showed marked hypoplasia at presentation. The three cases of drug induced hypoplastic marrow were secondary to phenytoin and carbamazepine for epilepsy, methotrexate therapy for rheumatoid arthritis and prolonged antithyroid medication for hyperthyroidism.
Pancytopenia in fanconi anaemia: a) Peripheral smear showing severe neutropenia (Leishman; 400X); b) Deformity of the hands in fanconi anaemia; c) Bone marrow biopsy with hypoplastic marrow (H&E, 40X); d) Pancytopenia in mucolipidosis: Liver biopsy of four month old child with extensive infiltration by storage cells (H&E, 400X).
Pancytopenia with Chronic Disorders
In the present study, CLD was an aetiological cause of pancytopenia in (n=57, 14.25%) of cases. An associated megaloblastic anaemia was seen in (n=14, 24.6%) of CLD patients, and, functional hypersplenism was noted in (n=8, 14%) cases of pancytopenia. The causes of CLD was found to be alcoholic liver disease (n=36, 9%), viral hepatitis (n=13, 3.25%), autoimmune hepatitis (n=13, 1%), non alcoholic steatohepatitis (n=3, 0.75%) and mucolipidosis (n=1, 0.25%). The liver biopsy in mucolipidosis revealed storage cell infiltration [Table/Fig-5d]. Primary idiopathic hypersplenism was diagnosed in two cases.
The haematological parameters of patients with pancytopenia revealed that the Hb was lowest in megaloblastic anaemia (4.62 gm/dL), lowest TLC in acute leukaemia (2077.93 cells) and lowest PC in retroviral disease (38,000 cells) and dengue fever (38,000 cells) compared to other causes and were also found statistically significant (p-value: 0.02, 0.045 and 0.001 respectively). High MCV of more than 100 was seen in megaloblastic anaemia (n=74, 79.22%), retroviral disease (n=9, 27.27%), myelodysplastic syndrome (n=2, 25%), aplastic anaemia (n=4, 17.39%), CLD (n=9, 15.78%) and acute leukaemia (n=2, 8%). An MCV of more than 99.6 fL was seen in (n=93, 100%) cases of megaloblastic anaemia. Various haematological parameters between megaloblastic and non megaloblastic causes of macrocytosis were statistically insignificant with a p-value of >0.05. Reticulocyte count and RPI were found statistically significant with a p-value of <0.05 and hypoplastic marrow presented with lowest reticulocyte count and RPI compared to other causes of pancytopenia.
The bone marrow aspiration was hypocellular in (n=34, 17%) cases, hyper cellular (n=124, 62%) and normocellular (n=8, 4%) and yielded only sinusoidal blood in (n=34, 17%) cases. In the present study, the common causes of hypocellular marrow were hypoplastic marrow (n=23, 11.5%) and myelofibrosis (n=8, 4%). Bone marrow biopsy evaluation was an important diagnostic tool to evaluate certain causes of pancytopenia such as aplastic anaemia, myelofibrosis and abnormal localisation of immature precursors in myelodysplastic syndrome.
Discussion
Pancytopenia can be detected when an abnormal clinical finding such as easy fatigability, infection or bleeding is subjected for further evaluation or sometimes, can even be spotted incidentally during routine checkups. It is a presenting feature in many diseases, and the severity and prognosis depends on the aetiological cause of pancytopenia. Differences in various methodology, stringency of criteria, topographical variations, and duration of observation and follow up, genetic variability and cytotoxic exposure directly influence the incidence of disorders leading to pancytopenia [3].
The common aetiologies for pancytopenia in the present study were malaria, megaloblastic anaemia, CLD, retroviral disease and acute leukaemia together accounting for (n=320, 80%) cases of pancytopenia with malaria and megaloblastic anaemia exclusively accounting for (n=201, 50.25%) of cases. In contrast, aplastic anaemia has been reported as the most common cause of pancytopenia throughout the world and this difference can be attributed to improved nutritional conditions and lower prevalence of infectious diseases in the west [5]. Malaria and megaloblastic anaemia can be easily detected and are the treatable causes and hence, should be reported promptly [6]. A diagnosis of megaloblastic anaemia does not warrant a bone marrow examination routinely, however, it may be performed to rule out other haematological causes if the diagnosis is not straightforward and in peripheral centers where folic acid and Vitamin B12 levels estimation are not available routinely [7]. In the literature, megaloblastic anaemia is the commonest cause of pancytopenia in India unlike the present study, where malaria constituted for (n=108, 27%) of cases which explains the endemicities of malaria in our region [3,6,7]. Bhatnagar SK et al., and Jain A and Naniwadekar M, have reported malaria as a cause of pancytopenia [8,9]. In a study by Hamid GA and Shukry SA, conducted in Yemen, the most common cause of pancytopenia was malaria (n=23, 30.6%) [4].
Similar to the present study, wide distribution of causes of pancytopenia were also encountered in various studies conducted in the past and results of age and sex distribution, and presenting complaints were similar [Table/Fig-6] [1,3-8,10,11]. In the present study, the age ranged widely (two days-90 years) and pancytopenia was seen even during neonatal period.
Comparison of common causes of pancytopenia among various studies.
| Study | Country | No of cases | MC* cause | Second MC* cause |
|---|
| Kumar R et al., [12] | India | 166 | Aplastic anaemia (30%) | Megaloblastic anaemia (22%) |
| Khodke K et al., [3] | India | 50 | Megaloblastic anaemia (44%) | Aplastic anaemia (14%) |
| Khunger JM et al., [6] | India | 144 | Megaloblastic anaemia (72%) | Aplastic anaemia (14%) |
| Jha A et al., [13] | Nepal | 148 | Aplastic anaemia (29.05%) | Megaloblastic anaemia (23.64%) |
| Gupta V et al., [15] | India | 155 | Aplastic anaemia (43%) | Acute leukaemia (25%) |
| Hamid GA and Shukry SA [4] | Yemen | 75 | Hypersplenism (28%) | Malaria (17.3%) |
| Santra G and Das BK [10] | India | 111 | Aplastic anaemia (22.72%) | Hypersplenism (13.51%) |
| Gayathri BN and Rao KS [1] | India | 104 | Megaloblastic anaemia (74.04%) | Aplastic anaemia (19%) |
| Makheja KD et al., [5] | Pakistan | 62 | Megaloblastic anaemia (41.9%) | Acute myeloid leukaemia (27.4%) |
| Jain A and Naniwadekar M [9] | India | 250 | Hypersplenism (29.2%) | Infections (25.6%) |
| Yokus O and Gedik H [7] | Istanbul | 137 | Vitamin B12 deficiency (17%) | Chronic liver disease (15%) |
| Present study | India | 400 | Malaria (27%) | Megaloblastic anaemia (23.25%) |
MC- Most common
Aplastic anaemia has been reported as one of the common aetiological cause of pancytopenia with a wide incident range of 10-52% [3,10,12,13]. Kumar R et al., has reported the highest incidence with (n=49, 29.5%) cases of hypoplastic marrow [12]. In a retrospective analysis of 118 patients with hypocellular marrow, Pol S et al., reported 61 patients with aplastic anaemia of undetermined cause, 19 patients with hepatitis induced aplasia and 38 patients with an inherited form [14]. In the present study, the common cause of hypoplastic marrow was idiopathic aplastic anaemia 13 (3.25%) cases.
Paediatric pancytopenia was seen in 68 (17%) cases in present study and malaria (n=23, 5.75%) was found to be the commonest cause of pancytopenia in paediatric age group as well. The other causes of paediatric pancytopenia were sepsis (n=17, 4.25%), acute leukaemia (n=12, 3%), hypoplastic marrow (n=7, 1.75%), CLD (n=3, 0.75%), megaloblastic anaemia (n=3, 0.75%), dengue fever (n=2, 0.5%), and a case of glycogen storage disorder (n=1, 0.25%). In a study conducted by Gayathri BN and Rao KS, paediatric pancytopenia accounted for (n=31, 29.8%) of cases with megaloblastic anaemia being the commonest cause followed by aplastic anaemia and similar outcomes were also reported by Bhatnagar SK et al., [1,8]. Gupta V et al., studied 105 patients aged 1.5 to 18 years presenting with pancytopenia and reported a mean age of 8.6 years [15]. In the same study, commoner causes of pancytopenia reported were aplastic anaemia (n=45, 43%) and acute leukaemia (n=26, 25%), and megaloblastic anaemia (n=7, 6.7%) [15].
An MCV of more than 99.6 fL was observed in 100% of megaloblastic anaemia cases thus, proving the sensitivity of the parameter in the diagnosis of megaloblastic anaemia. Hamid GA and Shukry SA, analysed MCV in various diseases causing pancytopenia and the results were comparable with the present study [Table/Fig-7] [4]. Macrocytosis in aplastic anaemia is a known fact [16]. A study conducted by Gupta PK et al., to differentiate aplastic anaemia and megaloblastic anaemia using red cell indices states that MCV in megaloblastic anaemia was higher in contrast to aplastic anaemia, where it was not statistically significant [16]. In addition, the presence of macroovalocytes and hypersegmented neutrophils in the former and the bone marrow biopsy findings in the latter will definitely help in differentiating the two entities [16]. The MCV will be increased secondary to CLD due to toxic effect of excess alcohol intake. Macrocytic anaemia is relatively common in patients with a myelodysplastic syndrome [17,18]. Macrocytosis, hypogranular neutrophils along with other features of dyshaematopoiesis and a normal serum levels of vitamin B12 and folic acid levels are found in peripheral blood and bone marrow of MDS [18].
Comparison of MCV of present study with Hamid GA and Shukry SA, [4].
| Diseases | MCV |
|---|
| Hamid GA and Shukry SA, [4] | Present study |
|---|
| Malaria | 80.8 | 81.5 |
| Aplastic anaemia | 88.4 | 91.4 |
| Megaloblastic anaemia | 101.2 | 100.5 |
| Myelofibrosis | 82.2 | 91.2 |
| Acute leukaemia | 86.1 | 89.7 |
| Myelodysplastic syndrome | 94 | 96.8 |
MCV- Mean corpuscular volume
The significance of bone marrow examination in evaluating the aetiology of pancytopenia has been well emphasised in earlier studies [19]. However, bone marrow examination is not a requirement in every pancytopenic patient, especially when a diagnosis can be made on a peripheral smear or the ancillary tests. However, in patients with sepsis, or bleeding when associated with significant neutropenia or thrombocytopenia, bone marrow evaluation is mandatory to rule out marrow suppression. Presence of blasts in peripheral smear, hypogranular or hyposegmented neutrophils, refractory cytopenias, unexplained splenomegaly, monoclonal gammapathy, or evaluation of metastatic tumours also requires bone marrow examination [12,19]. However, in megaloblastic anaemia and reactive marrow with obvious causes such as CLD, HIV and malaria bone marrow aspiration and biopsy may not provide any additional information and can be avoided. Simultaneous, bone marrow aspiration and biopsy should be performed where diagnosis is not straightforward that cannot be established using the other blood tests. While aspiration yields greater morphological details over biopsy, biopsy is superior to evaluate an accurate and more reliable cellularity index. It is noted that marrow infiltration, fibrosis and granulomas that are generally missed on aspiration can be easily detected on biopsy. Hence, aspiration and biopsy complement each other in providing a rapid yet precise diagnosis in the background of pancytopenia [12,19].
Limitation
The study is limited by lack of follow up in a small proportion of cases. A larger multicentric prospective study with emphasis on follow up for haematological recovery of blood counts may guide in understanding the clinical outcomes in various causes of pancytopenia.
Conclusion
This study highlights that in a developing country like India, where nutritional anaemia and aplastic anaemia are found to be the common causes for pancytopenia, malaria can be an important cause in the endemic regions, like ours. However, the management of pancytopenia is based on identifying and treating the underlying cause and also providing an adequate supportive therapy to deal with consequences of cytopenias. This necessitates a systematic approach in determining the cause since pancytopenia has a wide range of differential diagnosis. Awareness about the various causes, which is generally region specific and about the specific laboratory findings associated with each one, one can narrow down the differential diagnosis, guiding the clinician to initiate appropriate treatment modality and also to avoid unnecessary invasive procedures when not indicated.
M- Male, F-Female, AML-M2- Acute myeloblastic leukaemia with maturation, AML-M4- Acute myelomonocytic leukaemia, APL- Acute promyelocytic leukaemia, DLBCL- Diffuse large B-cell lymphoma, MDS- Myelodysplastic syndrome, HIV- Human immunodeficiency virus
MC- Most common
MCV- Mean corpuscular volume