Introduction
Type 2 Diabetes Mellitus (DM) is associated with hyperglycaemia, dyslipidaemia and oxidative stress. Oxidative damage, indicated by elevated levels of Malondialdehyde (MDA), plays a vital role in development of Coronary Artery Disease (CAD) in diabetics. Although, reduction of Low Density Lipoprotein (LDL) is identified as the primary target for therapy in dyslipidaemic patients by National Cholesterol Education Programme (NCEP), the levels of LDL may not be elevated in diabetic patients. Moreover, many studies have shown that apolipoprotein B100 and A1 (Apo B100 and Apo A1), which are the corresponding protein components of LDL and High Density Lipoproteins (HDL) respectively, are more sensitive markers for screening of CAD.
Aim
To determine the concentration of Apo A1 and Apo B100 along with serum MDA in patients with type 2 DM and study the correlation between them.
Materials and Methods
A case-control study was done on 75, type 2 diabetic patients and 75 healthy controls. Fasting plasma glucose, glycated haemoglobin, fasting lipid profile along with Apo A1, Apo B100 and MDA as a marker for lipid peroxidation were determined, compared and analysed using SPSS version 23.0.
Results
Diabetic patients had a significantly higher levels of serum triglyceride, Apo B100 and MDA along with significantly lower levels of HDL and Apo A1. The Apo B100/Apo A1 ratio was also higher in diabetic cases which showed a strong positive correlation with HbA1c (r=0.515) and MDA (r=0.819). The levels of total cholesterol and LDL were comparable in both the groups.
Conclusion
Diabetes mellitus is associated with dyslipidaemia and oxidative stress. Poor glycaemic control is associated with an increased oxidative damage which contributes to development of microvascular complication. Hence, Apo A1, Apo B100 and serum MDA may be included as additional parameters in the management of DM as they could help in early diagnosis and prevention of CAD.
Apolipoprotein A1, Apolipoprotein B100, Dyslipidaemia, Insulin resistance, Oxidative stress
Introduction
Diabetes mellitus has emerged as a global health issue. Currently, about 20-30 million people in India are diagnosed to have DM and this figure is expected to rise to about 80 million by 2030 [1,2]. Chronic hyperglycaemia seen in long standing DM, is responsible for various life threatening complications like retinopathy, nephropathy and atherosclerosis. Of these, CAD or cardiovascular complications are now established as a major factor for increased morbidity and mortality in diabetics. Epidemiologic studies have also demonstrated that DM is an independent risk factor for CAD [3]. Along with smoking, hypertension and other factors, dyslipidaemia in the presence of hyperglycaemia accelerates the incidence of cardiovascular disease [4]. The NCEP in their Adult Treatment Plan (ATP) III has identified high LDL level to be the major risk factor for CAD [5]. Though dyslipidaemia is a common presentation in chronic diabetics, the plasma levels of LDL may not be increased in all diabetics [6,7]. HDL and LDL consists of a protein component called as Apo A1 and Apo B100 respectively. Recent studies suggest that plasma concentration of Apo A1 and Apo B100 and their ratio (Apo B100/Apo A1 ratio) are more sensitive and specific biochemical markers for the risk of CHD than the conventional lipid and lipoprotein measurement [8-12].
Oxidative stress, defined as an imbalance between the Reactive Oxygen Species (ROS) and the antioxidant, plays an important role in the development of diabetic complication. Excessive production of ROS results in peroxidation of lipids which is abundantly present in the cellular membrane. This mechanism is suspected to play an important role in pathogenesis of diabetic complication including atherosclerosis and CAD [13]. MDA is a stable and easily measurable product of lipid peroxidation. They are frequently used to evaluate the oxidative stress [14]. Although, microvascular and macrovascular complications of DM are known to increase with DM severity, the association between DM and MDA levels in type 2 DM remains controversial and contradictory as both increase and decrease of MDA have been reported in DM [14].
Hence, this study was carried out to determine the Apo A1 and Apo B100 levels along with serum MDA levels and study the effect of type 2 DM on these parameter and establish a correlation between them.
Materials and Methods
This case-control study was carried out at a tertiary care teaching hospital in North India after obtaining Institutional Ethical Committee clearance. Between July 2016 to December 2016, a total of 150 participant who had given their consent were included in the study. The participants were divided into two Groups-A and B. Group A included 75 newly diagnosed cases of type 2 DM and Group B included 75 healthy controls who were matched for age and gender. Smokers, hypertensives, patients with existing or past history of cardiac disease, hepatic or renal disorder and those on treatment with lipid lowering agents were excluded.
Biochemical Determination
Under aseptic condition 1 mL of venous blood was collected in a plain serum collecting vacutainer and 2 mL venous blood was collected in an EDTA containing vacutainer and a sodium fluoride vacutainer from the participants of both the groups in a fasting state. The sample collected in plain vacutainer was allowed to clot for 30 minutes and then centrifuged at 2000 gm for 10 minutes for the separation of serum. The blood collected in the sodium fluoride vacutainer was mixed gently and then centrifuged to separate the plasma. Plasma sample was used for determining the fasting plasma glucose levels by Glucose Oxidase-Peroxidase (GOD-POD) method [15]. Glycated haemoglobin (HbA1c) was determined by enhanced particle immunoturbidimetric method using the whole blood sample collected in Ethylenediaminetetraacetic Acid (EDTA) vacutainer [16]. Serum samples were used to determine the total cholesterol by Cholesterol Oxidase-Phenol 4-Aminoantipyrine Peroxidase (CHOD-PAP) method, serum triglyceride by Glycerol Phosphate Oxidase-Phenol 4-Aminoantipyrine Peroxidase (GPO-PAP) method), HDL (enzymatic method), LDL (enzymatic method), Apo A1 (immumoturbidimetric method) and Apo B100 (immunoturbidimetric method) [17-19]. Fasting plasma glucose, Glycated Hb (HbA1c) and lipid profile were all determined using ErBa 360 autoanalyser. Apo A1 and Apo B100 were determined using autoanalyser. MDA was determined from the serum sample by Thiobarbituric Acid (TBA) method using a spectrophotometer at 532 nm [20].
Statistical Analysis
All the values obtained were compiled and analysed using Statistical Package for Social Sciences Version 23.0 (SPSS/PC; SPSS-23.0, Chicago, USA). The values were expressed as mean±SD. Independent ‘t’-test was used to study the significance of the means obtained. Correlation and regression were applied for analysis of association between the variables. Tables and charts were created using Microsoft Excel. A p-value <0.05 was considered significant.
Results
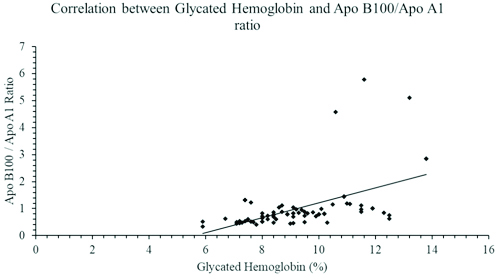
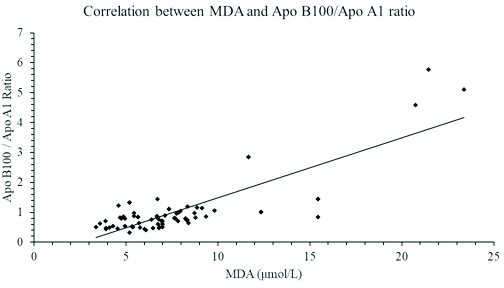
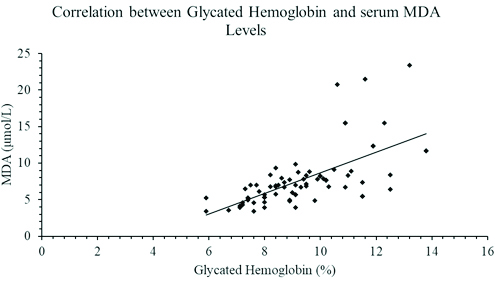
As seen in [Table/Fig-1], the study included 48 diabetic males and 27 diabetic females. The mean age of diabetic patients was 53.36±8.64 years. The lipid profile of diabetic patients was deranged when compared with healthy controls. The increased levels of serum triglyceride, Apo B100 and serum MDA was statistically significant with a p-value of <0.001, <0.04 and <0.001 respectively. The HDL and Apo A1 level were significantly lower (p-value <0.001) in the diabetic cases. The diabetic cases also showed a significantly elevated Apo B100/Apo A1 ratio (p=0.001) as compared to healthy controls. The changes observed among the cases and controls with regards to total cholesterol and LDL were comparable. Apo B100/Apo A1 ratio showed a strong significant positive correlations with HbA1c and MDA levels with Pearson’s coefficient of r=0.515 [Table/Fig-2] and r=0.819 [Table/Fig-3] respectively. As seen in [Table/Fig-4], a significant positive correlation was also observed between HbA1c and serum MDA level (r=0.641; p<0.001).
Findings showing the gender distribution, age, fasting lipid profile, glycated haemoglobin, apolipoproteins and MDA levels in type 2 diabetic cases and control. The significance of mean difference was determined by using Independent ‘t’-test.
| Variables | Controls (n=75) | Cases (n=75) |
|---|
| Males | 40 | 48 |
| Females | 35 | 27 |
| Age (Years) (mean±SD) | 52.04±12.54 | 53.36±8.64 |
| Fasting plasma glucose (mg/dL) (mean±SD) | 95.64±14.77 | 197.45±64.07* |
| Glycated haemoglobin (%) (mean±SD) | 5.96±0.89 | 9.39+1.80* |
| Triglyceride (mg/dL) (mean±SD) | 128.35±72.48 | 229.68±96.52* |
| Total cholesterol (mg/dL) (mean±SD) | 179.95±46.63 | 187.32±40.19 |
| HDL (mg/dL) (mean±SD) | 46.16±14.62 | 33.90±10.29* |
| LDL (mg/dL) (mean±SD) | 109.41±39.24 | 112.17±38.11 |
| Apolipoprotein-A1 (mg/dL) (mean±SD) | 134.13±31.51 | 108.41±33.49* |
| Apolipoprotein-B100 (mg/dL) (mean±SD) | 80.56±22.89 | 88.32±22.95** |
| Apo-B/Apo-AI ratio (mean±SD) | 0.63±0.21 | 1.03±0.95*** |
| MDA (μmol/L) (mean±SD) | 3.73±1.10 | 7.71±3.68* |
(p-value <0.05 was considered significant)
* p<0.001 ** p=0.004 ***p=0.001
A graph showing a Pearson’s correlation coefficient between glycated haemoglobin and Apo B100/Apo A1 ratio in patients with type 2 diabetes mellitus (r=0.515; p<0.001).
A graph showing a Pearson’s correlation coefficient between Apo B100/Apo A1 ratio and Serum MDA levels in patients with type 2 diabetes mellitus (r=0.819; p<0.001).
A graph showing a Pearson’s correlation coefficient between glycated haemoglobin and serum MDA levels in patients with type 2 diabetes mellitus (r=0.641; p<0.001).
Discussion
Diabetes mellitus is associated with a deranged metabolism of both glucose and lipids resulting in hyperglycaemia and free fatty acidaemia. Moreover, the release of metabolic mediators such as cytokines from the adipose tissue along with other pro-oxidants induces a state of oxidative stress which are responsible for diabetic complications [21]. Oxidation of polyunsaturated fatty acid results in production of MDA which is used as a marker to assess oxidative damage. Therefore, this research was conducted to study the effect of type 2 DM on the basic lipid profile along with the apolipoproteins and also on the production of MDA. The mean age of the diabetic patients in this study was in accordance with earlier studies such as Kaveeshwar SA and Cornwall J, Cheema A et al., in their meta-analysis, they reported that type 2 DM was highest in the fifth decade of life [1,2].
Diabetic patients have deranged lipid profile and is characterised by lipid triad i.e., high serum triglyceride (TAG), low HDL levels and elevated small dense LDL particles [22,23]. One of the most characteristic finding in diabetic patient is hypertriglyceridaemia. This study was in accordance as diabetic patients showed significantly high serum TAG levels (p<0.001) and low HDL levels (p<0.001).
Insulin resistance is associated with an elevated levels of TAG rich Very Low Density Lipoprotein (VLDL). This is a result of increased activity of Hormone Sensitive Lipase (HSL) in the adipose tissue and decreased TAG catabolism by lipoprotein lipase in the liver [24,25]. The elevated TAG has a direct action on HDL as they enhance the enzymatic activity of Cholesteryl Ester Transfer Protein (CETP) which catalyses the transfer of TAG from VLDL to HDL in exchange of cholesteryl esters. These TAG rich HDL undergoes increased catabolism thus reducing their circulating concentration [26,27].
The LDL is identified as the primary target for therapy by the NCEP in their ATP III guidelines [5,28]. Though many studies have shown elevated levels of circulatory LDL, patients with type 2 DM may not necessarily have a higher LDL concentration when compared with nondiabetic individuals [6,7]. Many literature researches have reported that rather than the concentration, it is the size and the number of the LDL particle that influences its atherogenic activity which makes a diabetic patient prone to develop CAD [29]. Small dense LDL particle are more atherogenic than larger LDLs as small dense LDLs are removed by scavenger cells which is a critical step in the development of atherosclerosis [30]. In the present study, no significant increase in the LDL levels were observed in diabetic cases which was in accordance with other studies. Sultania S et al., in a study on 50 diabetic patients, observed no significant increase in the LDL concentration when compared with healthy controls [31]. Similarly Zeqollari A et al., in a study on 102 diabetic patients observed that the mean LDL concentration was 118.37 mg/dL, with only 17% type 2 diabetic patients having LDL levels above 160 mg/dL [32].
Apolipoproteins are the protein components which binds with phospholipids to form a surface monolayer in all mature lipoprotein particles. Apo A1 and Apo B 100 are the major apolipoprotein components of antiatherogenic HDL and proatherogenic LDL respectively [8]. Although, LDL and non HDL lipoproteins are considered as important prognostic indicators for the development of CAD, many studies has suggested that apolipoproteins maybe better markers for predicting CAD. The five year prospective Quebec cardiovascular study concluded that for each standard deviation increase in baseline Apo B, there was a significant increase in relative risk for CHD [9]. Caslake MJ et al., reported that though the LDL levels did not differ much between the CAD patients and the healthy controls, the disease group had a significantly higher Apo B levels [10]. The Prospective Epidemiological Study of Myocardial Infarction (PRIME) study, assessed the predictive ability of HDL and its constituent in the development of CHD and concluded that Apo A1 was the strongest predictor of CHD [11]. A cohort study called as Apolipoprotein-related Mortility Risk (AMORIS) study, confirmed Apo A1, Apo B and Apo B/Apo A1 ratio as stronger risk factors for development of coronary heart disease [12]. In the present study, the diabetic patients had a significantly lower Apo A1 levels (p-value <0.001) which correlated well with their corresponding HDL. It was also observed that even though the LDL levels did not show any significant increase in diabetic patients, their Apo B 100 were significantly elevated (p=0.004). The Apo B100/Apo A1 ratio which represents the balance between proatherogenic and anti atherogenic lipoproteins was significantly increased in diabetic cases (p=0.001). A strong correlation between the Apo B100/Apo A1 ratio and HbA1c (r=0.515; p-value <0.001) indicates that glycemic control has a direct effect of incidence of CAD.
The ROS produced during the normal course of metabolic pathways are effectively counteracted by production of cellular antioxidants [33]. Many research have established a link between DM and increased production of ROS, resulting in oxidative stress which has detrimental effect resulting in β cell dysfunction, dyslipidaemia and insulin resistance [13,34]. Glucose auto-oxidation, polyol pathway, increased activity of Protein kinase C, increased production of Advance Glycated End product (AGE) and increased expression for its receptors have been postulated to cause oxidative damage [35].
Polyunsaturated fatty acids undergoes oxidative damage to produce MDA. High levels of MDA in diabetic patients is associated with cardiovascular disease [36]. According to study reported by Rankinen T et al., high plasma MDA levels correlated with elevated fibrinogen level in middle age men [37]. In another study done by Pasaoglu H et al., high erythrocyte MDA activity was reported in diabetic patients [14]. The findings in this study was as per the findings of the above studies. Diabetic patients had a significantly higher levels of MDA in comparison to the control. The degree of lipid peroxidation correlated strongly with both Apo B100/Apo A1 ratio (r=0.819; p-value <0.001) indicating that with poor glucose control is associated with higher oxidative damage.
Limitation
In future, further large scale prospective multicentric or community based study would be required to establish the role of MDA and apolipoproteins levels in the management and prevention of CAD in type 2 DM. Other factors such as sedentary lifestyle, diet and work related stress which are known to affect the lipid profile were not considered in this study.
Conclusion
It is evident from this study that DM is associated with dyslipidaemia and oxidative stress. Apo A1 and Apo B100 are being preferred as an additional marker to evaluate the dyslipidaemia. The Apo B100/Apo A1 ratio in diabetic patients were elevated and showed significant positive correlation with both HbA1c and MDA. This indicates that poor glycemic control is associated with increased oxidative damage which contributes to development of microvascular complication. Hence, determining MDA levels and Apo B100/Apo A1 ratio during the management of DM could help in early diagnosis of diabetic complication and effectively manage them. However, a large scale study with other parameter of CAD such as angiography is required to either accept our claim or refute it.
(p-value <0.05 was considered significant)
* p<0.001 ** p=0.004 ***p=0.001
[1]. Kaveeshwar SA, Cornwall J, The current state of diabetes mellitus in IndiaAustralas Med J 2014 7:45-48.10.4066/AMJ.2014.1979 [Google Scholar] [CrossRef]
[2]. Cheema A, Adeloye D, Sidhu S, Sridhar D, Chan KY, Urbanization and prevalence of type 2 diabetes in Southern Asia: a systematic analysisJ Glob Health 2014 4(1):01040410.7189/jogh.04.01040424976963 [Google Scholar] [CrossRef] [PubMed]
[3]. Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, de Boer IH, Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the american heart association and the american diabetes associationDiabetes Care 2015 132:691-718.10.1161/CIR.000000000000023026246173 [Google Scholar] [CrossRef] [PubMed]
[4]. Mooradian AD, Dyslipidemia in type 2 diabetes mellitusNat Clin Pract Endocrinol Metab 2009 5(3):150-59.10.1038/ncpendmet106619229235 [Google Scholar] [CrossRef] [PubMed]
[5]. Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii). final report. national cholesterol education program. NIH 2012Circulation 2002 106(25):3143-421.10.1161/circ.106.25.314312485966 [Google Scholar] [CrossRef] [PubMed]
[6]. Wu L, Parhofer KG, Diabetic dyslipidemiaMetabolism 2014 63(12):1469-79.10.1016/j.metabol.2014.08.01025242435 [Google Scholar] [CrossRef] [PubMed]
[7]. Vergès B, Pathophysiology of diabetic dyslipidaemia: where are we?Diabetologia 2015 58(5):886-99.10.1007/s00125-015-3525-825725623 [Google Scholar] [CrossRef] [PubMed]
[8]. Murray RK, Bender D, Botham KM, Harper’s Illustrated Biochemistry 2012 Apr 1 29th edNew YorkMcGraw-Hill MedicalISBN:9780071765763 [Google Scholar]
[9]. Lamarche B, Moorjani S, Lupien PJ, Cantin B, Bernard PM, Dagenais GR, Apolipoprotein A-I and B levels and the risk of ischemic heart disease during a five-year follow-up of men in the Québec cardiovascular studyCirculation 1996 94(3):273-78.10.1161/01.CIR.94.3.2738759066 [Google Scholar] [CrossRef] [PubMed]
[10]. Caslake MJ, Packard CJ, Suckling KE, Holmes SD, Chamberlain P, Macphee CH, Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase: a potential new risk factor for coronary artery diseaseAtherosclerosis 2000 150:413-19.10.1016/S0021-9150(99)00406-2 [Google Scholar] [CrossRef]
[11]. Luc G, Bard JM, Ferrieres J, Evans A, Amouyel P, Arveiler D, Value of HDL cholesterol, apolipoprotein A-I, lipoprotein A-I, and lipoprotein A-I/A-II in prediction of coronary heart disease the prime study. Prospective epidemiological study of myocardinal infractionArterioscler Thromb Vasc Biol 2002 22(7):1155-61.10.1161/01.ATV.0000022850.59845.E012117731 [Google Scholar] [CrossRef] [PubMed]
[12]. Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E, High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective studyLancet 2001 358(9298):2026-33.10.1016/S0140-6736(01)07098-2 [Google Scholar] [CrossRef]
[13]. Tangvarasittichai S, Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitusWorld J Diabetes 2015 6(3):456-80.10.4239/wjd.v6.i3.45625897356 [Google Scholar] [CrossRef] [PubMed]
[14]. Pasaoglu H, Sancak B, Bukan N, Lipid peroxidation and resistance to oxidation in patients with type 2 diabetes mellitusTohoku J Exp Med 2004 203:211-18.10.1620/tjem.203.21115240931 [Google Scholar] [CrossRef] [PubMed]
[15]. Trinder P, Enzymatic colorimetric method for glucose determinationAnn ClinBiochem 1969 6:24-7.[Available from]: http://journals.sagepub.com/doi/pdf/10.1177/000456326900600108 [Google Scholar]
[16]. Jeppsson JO, Kobold U, Barr J, Finke A, Hoelzel W, Hoshino T, Approved IFCC reference method for the measurement of HbA1c in human bloodClin Chem Lab Med 2002 40(1):78-89.10.1515/CCLM.2002.01611916276 [Google Scholar] [CrossRef] [PubMed]
[17]. Roeschlau P, Bernt E, Gruber W, Enzymatic determination of total cholesterol in serumZ Klin Chem Klin Biochem 1974 12(5):226 [Google Scholar]
[18]. McGowan MW, Artiss JD, Strandbergh DR, Zak B, A peroxidase-coupled method for the colorimetric determination of serum triglyceridesClin Chem 1983 29(3):538-42. [Google Scholar]
[19]. Burtis C, Ashwood E, In: tietz textbook of clinical chemistry and molecular diagnostics, 5th ed.,ch 27 2012 LondonElsevier Health SciencesLipids, lipoproteins, apolipoproteins, and other cardiovascular risk factors. pp.785-86 [Google Scholar]
[20]. D’souza D, Subhas BG, Shetty SR, Balan P, Estimation of serum malondialdehyde in potentially malignant disorders and post-antioxidant treated patients: a biochemical studyContemp Clin Dent 2012 3(4):448-51.10.4103/0976-237X.10743823633807 [Google Scholar] [CrossRef] [PubMed]
[21]. Cersosimo E, Triplitt C, Mandarino LJ, DeFronzo RA, Pathogenesis of Type 2 Diabetes Mellitus. In: De Groot LJ, Chrousos G, Dungan K, et al. editorsEndotext [Internet] 2000-2015 South Dartmouth (MA)MDText.com, Inc.Last Update: May 28, 2015 [Google Scholar]
[22]. Revathy K, Arunkumar D, Gandhi M, Swaminathan S, Association between atherogenic ratio to fasting plasma glucose and HbA1cInt J Pharm Sci Res 2016 7(5):2136-41. [Google Scholar]
[23]. Mancinelli K, The Ketogenic Diet: A Scientifically Proven Approach to Fast, Healthy Weight Loss 2014 Dec 22 Ulysses Press [Google Scholar]
[24]. Hogan MF, Hull RL, The islet endothelial cell: a novel contributor to beta cell secretory dysfunction in diabetesDiabetologia 2017 60(6):952-59.10.1007/s00125-017-4272-928396983 [Google Scholar] [CrossRef] [PubMed]
[25]. Arca M, Pigna G, Favoccia C, Mechanisms of diabetic dyslipidemia: relevance for atherogenesisCurr Vasc Pharmacol 2012 10(6):684-86.10.2174/15701611280352086423259553 [Google Scholar] [CrossRef] [PubMed]
[26]. Lorenzo C, Hartnett S, Hanley AJ, Rewers MJ, Wagenknecht LE, Karter AJ, Impaired fasting glucose and impaired glucose tolerance have distinct lipoprotein and apolipoprotein changes: the insulin resistance atherosclerosis studyJ Clin Endocrinol Metab 2013 98(4):1622-30.10.1210/jc.2012-318523450048 [Google Scholar] [CrossRef] [PubMed]
[27]. Aggarwala J, Sharma S, Saroochi Ajay J, Sarkar A, Effects of aerobic exercise on blood glucose levels and lipid profile in Diabetes Mellitus type 2 subjectsAl Ameen J Med Sci 2016 9(1):65-69. [Google Scholar]
[28]. Grundy SM, Becker D, Clark LT, Cooper RS, Denke MA, Howard WJ, Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III): Final reportCirculation 2002 106:3142-373.10.1161/circ.106.25.314312485966 [Google Scholar] [CrossRef] [PubMed]
[29]. Wang J, Stancakova A, Soininen P, Kangas AJ, Paananen J, Kuusisto J, Lipoprotein subclass profiles in individuals with varying degrees of glucose tolerance: a population-based study of 9399 Finnish menJ Intern Med 2012 272:562-72.10.1111/j.1365-2796.2012.02562.x22650159 [Google Scholar] [CrossRef] [PubMed]
[30]. Huang YC, Chang PY, Hwang JS, Ning HC, Association of small dense lowdensity lipoprotein cholesterol in type 2 diabetics with coronary artery diseaseBiomed J 2014 37(6):375-79.10.1364/BIOMED.2014.BT3A.7525179702 [Google Scholar] [CrossRef] [PubMed]
[31]. Sultania S, Thakur D, Kulshreshtha M, Study of lipid profile in type 2 diabetes mellitus patients and its correlation with HbA1cInt J Contemp Med Res 2017 4(2):437-39. [Google Scholar]
[32]. Zeqollari A, Spahiu K, Vyshka G, Çakërri L, Lipid profile in diabetes mellitus type 2 patients in albania and the correlation with BMI, hypertension, and hepatosteatosisJ Family Med Community Health 2014 1(4):1018[Available from] https://www.jscimedcentral.com/FamilyMedicine/familymedicine-1-1018.pdf [Google Scholar]
[33]. Cossarizza A, Ferraresi R, Troiano L, Roat E, Gibellini L, Bertoncelli L, Simultaneous analysis of reactive oxygen species and reduced glutathione content in living cells by polychromatic flow cytometryNat Protoc 2009 4:1790-97.10.1038/nprot.2009.18920010930 [Google Scholar] [CrossRef] [PubMed]
[34]. Bonomini F, Rodella LF, Rezzani R, Metabolic syndrome, aging and involvement of oxidative stressAging Dis 2015 6(2):109-20.10.14336/AD.2014.030525821639 [Google Scholar] [CrossRef] [PubMed]
[35]. Rahimi-Madiseh M, Malekpour-Tehrani A, Bahmani M, Rafieian-Kopaei M, The research and development on the antioxidants in prevention of diabetic complicationsAsian Pac J Trop Med 2016 9(9):825-31.10.1016/j.apjtm.2016.07.00127633293 [Google Scholar] [CrossRef] [PubMed]
[36]. Tangvarasittichai S, Poonsub P, Tangvarasittichai O, Sirigulsatien V, Serum levels of malondialdehyde in type 2 diabetes mellitus Thai subjectsMed J 2009 61:20-23. [Google Scholar]
[37]. Rankinen T, Hietanen E, Väisänen S, Lehtiö M, Penttilä I, Bouchard C, Relationship between lipid peroxidation and plasma fibrinogen in middle-aged menThromb Res 2000 99:453-59.10.1016/S0049-3848(00)00271-1 [Google Scholar] [CrossRef]