Para-Substituted Functionalised Ferrocene Esters with Novel Antibacterial Properties
Kevin Muñoz Forti1, Faviola Bernard2, Gustavo Santiago-Collazo3, Waldemar Garcia4, Jose L Vera5, Enrique Meléndez6, Edu B Suarez-Martinez7
1 Graduate Student, Department of Biotechnology, Pontifical Catholic University of Puerto Rico, Ponce PR 00731; Research Coordinator, Department of Biology, University of Puerto Rico, Ponce PR 00716.
2 Student, Department of Biology, University of Puerto Rico, Ponce PR 00716.
3 Student, Department of Biology, University of Puerto Rico, Ponce PR 00716.
4 Technician, Department of Biology, University of Puerto Rico, Ponce PR 00716.
5 Professor, Department of Chemistry, University of Puerto Rico, Mayagüez PR 00681; Inter American University of San German Biology, Chemistry, and Environmental Science Department Calle Luna, San Germán 00683.
6 Professor, Inter American University of San German Biology, Chemistry, and Environmental Science Department Calle Luna, San Germán 00683.
7 Professor, Department of Biology, University of Puerto Rico, Ponce PR 00716; Professor, Ponce Research Institute, Ponce Health Sciences University, Ponce, Puerto Rico 00732.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Edu B Suarez-Martinez, Professor, Department of Biology, University of Puerto Rico, Ponce PR 00716; Professor, Ponce Research institute, Ponce Health Sciences University, Ponce, Puerto Rico 00732.
E-mail: edu.suarez@upr.edu
Introduction
Bacterial antibiotic resistance is on rise despite advances in the development of new antibiotics. In an attempt to circumvent resistance, scientists are shifting focus from modifying existent antibiotics to identifying new antibiotic compounds.
Aim
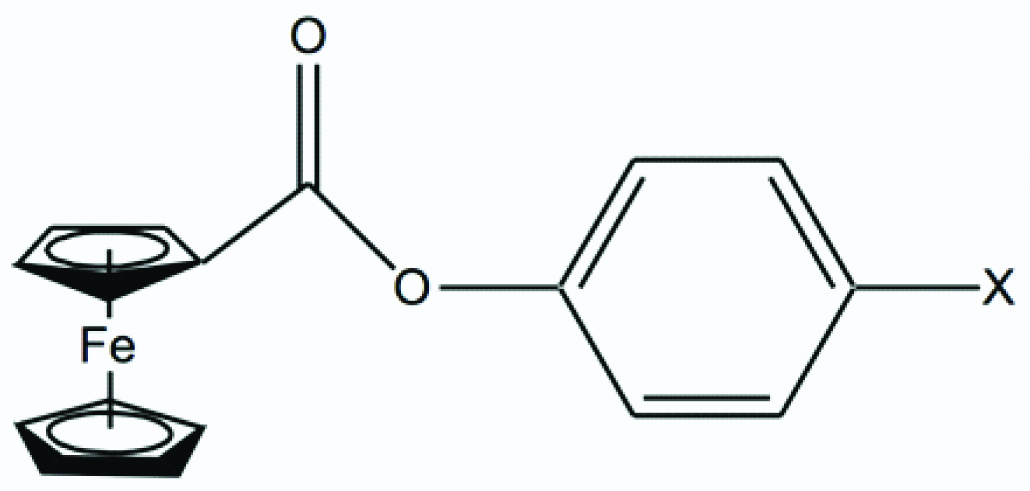
To assess the potential antibiotic effects of functionalised ferrocenecarboxylates para-substituted on the phenoxy pendant group to form: 4-fluorophenyl, 4-chlorophenyl, 4-bromophenyl, 4-iodophenyl and 4-(H-pyrrol-1-yl)phenyl.
Materials and Methods
For this, we employed the Kirby-Bauer disc diffusion method using a collection of nine bacterial species: Staphylococcus aureus, Escherichia coli, Micrococcus luteus, Pseudomonas aeruginosa, Serratia marcescens, Klebsiella pneumoniae, Bacillus subtilis, Proteus vulgaris and Enterobacter aerogenes.
Results
The results show that all four-halogen substituted ferrocenecarboxylates 4-fluorophenyl (23.33 μM, 11.66 μM, 5.83 μM), 4-chlorophenyl (10.16 μM, 5.08 μM, 2.54 μM), 4-bromophenyl (9.0 μM, 4.5 μM, 2.25 μM), and 4-iodophenyl (17.12 μM, 8.56 μM, 4.28 μM) exhibited an antibacterial effect by reducing proliferation of Bacillus subtilis. Meanwhile, only 4-bromophenyl (9.0 μM) and 4-chlorophenyl (10.16 μM) ferrocenecarboxylates were able to decrease the growth of Micrococcus luteus.
Conclusion
Hence, functionalised ferrocenecarboxylates para-substituted with small and simple groups represent a novel class of bio-organometallic compounds with the potential to be used as antibacterial agents.
Antibacterial, Bio-organometallic compounds, Phenoxy pendant, Ferrocenecarbolylate
Introduction
The over use of antibiotics and accelerated microevolution of bacteria have created a major threat to healthcare. Bacteria have developed antibiotic resistance genes through random mutations and have capitalised on horizontal gene transfer to facilitate the creation of bacteria strains with de novo antibiotic resistance [1]. Resistance to one antibiotic has even been shown to influence how a bacterium responds to another non related antibiotic [2]. Furthermore, it has been shown that Gram positive bacteria have developed resistance to antibiotics prior to their commercialisation via non anthropological exposure [3]. Bio-organometallic chemistry is developing a myriad of alternatives with compounds exhibiting unprecedented modes of actions and potential of combating antibiotic-resistant bacterial strains. It has been shown that the modification of organometallics with existing antibiotics may be able to overcome antibiotic resistance [4]. For instance, organometallic structures such as functionalised ferrocenes, exhibit a combination of biocompatibility, lipophilicity and reduction-oxidation (redox) properties that hold great promise in biomedicine. These structures present a platform for scaffolding and functionalisation of fathomless possibilities, some of which have already been shown to be successful novel antitumour, anti-parasitic, antifungal, and antimalarial alternatives [5-10]. For example, ferroquine, a ferrocene derivative of the drug chloroquine, has shown to overcome the drug resistance developed by malarial parasites to common antimalarial drugs [11]. However, despite the aforementioned advances in bio-organometallic research, studies evaluating the antibacterial activity of ferrocenecarboxylates are scarce. This study focused on the antibacterial effect of the following functionalised ferrocenes para-substituent on the phenoxy pendant group [Table/Fig-1]: 4-fluorophenyl-ferrocenecarboxylate (Fc-4F-Ph), 4-chlorophenyl-ferrocenecarboxylate (Fc-4Cl-Ph), 4-bromophenyl-ferrocenecarboxylate (Fc-4Br-Ph), 4-iodophenyl-ferrocenecarboxylate (Fc-4I-Ph), and their analog replacing the halide with a redox active species 4-(1H-pyrrol-1-yl)phenyl ferrocenecarboxylate (Fc-Py). The Kirby-Bauer disc diffusion susceptibility method was used to assess possible antibacterial activity of functionalised ferrocenes on Staphylococcus aureus, Escherichia coli, Micrococcus luteus, Pseudomonas aeruginosa, Serratia marcescens, Klebsiella pneumoniae, Bacillus subtilis, Proteus vulgaris and Enterobacter aerogenes.
Structure of ferrocenecarboxylate esters. “X” of the phenoxypendant group indicates substitution site X=F, Cl, Br, I, or Py.
This study represents the first of its kind, reporting the antibacterial properties of para-substituted ferrocenecarboxylates using a diverse gamut of microorganisms. The studied organisms encompass both Gram-positive and Gram-negative bacteria from various genera, of varied morphologies and metabolisms. These compounds may represent alternatives for treatment of antibiotic resistant strains and possibly reduce their burden on public health systems.
Materials and Methods
The present in vitro study was conducted from August 2015 to May 2016 at the Biosafety Level 2 PRISE Research laboratory, Department of Biology at the University of Puerto Rico in Ponce.
Bacterial Cultures
Nine bacterial species were tested. These were inoculated on solid growth media and cultured in 100 mm petri dishes under their respective conditions and nutritional requirements [Table/Fig-2]. These organisms were obtained from ATCC commercial strains, previously maintained and characterised by the Department of Biology, University of Puerto Rico, Ponce.
The culturing conditions and antibiotic control used in study for each bacterium.
| Organism | Agar Media | Temperature (°C) | Incubation Time (hours) | Antibiotic Concentration |
|---|
| S. aureus ATCC® 6538TM | Mannitol salt | 37°0 | 24 | Penicillin (500 I.U./mL) |
| E. coli ATCC® 25822TM | Luria Bertani | 37°0 | 24 | Kanamycin (50 μg/mL) |
| M. luteus ATCC® 10240TM | Nutrient | 30°0 | 72 | Penicillin (500 I.U./mL) |
| P. aeruginosa ATCC® 27853TM | Pseudomonas | 37°0 | 24 | Kanamycin (50 μg/mL) |
| S. marcescens ATCC® 14756TM | Nutrient | 25°0 | 48 | Kanamycin (50 μg/mL) |
| K. pneumoniae ATCC® 13883TM | MacConkey | 37°0 | 24 | Kanamycin (50 μg/mL) |
| E. aerogenes ATCC® 13048TM | Nutrient | 30°0 | 24 | Kanamycin (50 μg/mL) |
| P. vulgaris ATCC® 8427TM | MacConkey | 37°0 | 48 | Kanamycin (50 μg/mL) |
| B. subtilis ATCC® 6633TM | Nutrient | 30°0 | 24 | Kanamycin (50 μg/mL) |
Staphylococcus aureus, Escherichia coli, Micrococcusluteus, Pseudomonas aeruginosa, Serratia marcescens, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus vulgaris and Bacillus subtilis.
Synthesis of Ferrocene Esters
The ferrocene esters used in this study 4-fluorophenyl, 4-chlorophenyl, 4-bromo, 4-iodo, and 4-(1H-pyrrol-1-yl)-phenyl were synthesised and characterised as previously detailed [11]. In summary, ferrocenecarboxylic acid was dissolved in dry dichloromethane at room temperature under a nitrogen atmosphere. To this solution, oxalyl chloride was added drop wise and stirred overnight and the resulting solution was filtered. Separately, under a nitrogen atmosphere the para-substituted phenol vector was dissolved in dichloromethane; the ferrocenecarbonyl chloride filtrate previously prepared, was added drop wise to this solution while it stirred over the course of 6-12 hours. The reaction was monitored using Thin Layer Chromatography (TLC) and the mixture was filtered through a celite pad. The filtrate was washed with 0.1M HCl and purified by column chromatography using silica as the stationary phase and dichloromethane as the mobile phase. Characterisation was performed by Nuclear Magnetic Resonance (NMR) spectroscopy, Infrared (IR) spectroscopy, and elementary analysis.
Ferrocenecarboxylates Treatments
The six ferrocenes compounds tested; Fc-4Br-Ph, Fc-4Cl-Ph, Fc-4F-Ph, Fc-4I-Ph, Fc-4Py-Ph, and Fc-Ph were prepared by suspending a total of 1.3 mg of each compound in 150 μL of Dimethyl Sulfoxide (DMSO) (vehicle) and then agitated at 1,200 rpm for one hour at room temperature. After which the compounds were diluted with sterile cell culture grade water to their final concentrations listed in [Table/Fig-3].
Ferrocene compounds with the concentrations used in this study expressed in μM.
| Ferrocenes Compounds | Concentration I μM | Concentration II μM | Concentration III μM |
|---|
| Fc-4F-Ph | 23.33 | 11.66 | 5.83 |
| Fc-4Br-Ph | 9.00 | 4.50 | 2.25 |
| Fc-4I-Ph | 17.12 | 8.56 | 4.28 |
| Fc-4Cl-Ph | 10.16 | 5.08 | 2.54 |
| Fc-4Py-Ph | 9.04 | 4.52 | 2.26 |
| Fc-Ph | 24.80 | 12.40 | 6.20 |
Kirby-Bauer Disc Diffusion
Compounds were first screened to optimise volume and conditions with one positive control and one disc per compound concentration. For experiments each petri plate was divided into five sections including one as a negative vehicle control (DMSO+cell culture grade water), one with an antibiotic for positive inhibition control (varies by organism) and three for each concentration (I, II, and III) of the six ferrocenes compounds tested [Table/Fig-4]. A total of 50 μL of broth media containing active bacteria cultures were used to inoculate each plate. Thirty minutes after inoculation, sterile filter paper discs with a diameter of 0.63 mm were placed in triplicates for each compound concentration in their appropriate sections and a single disc was placed in the control sections. This was followed by the addition 10 μL of each corresponding ferrocene or control solution directly to the center of the filter disc. The plates were incubated under the conditions specified in [Table/Fig-2], the plates were evaluated for bacterial growth inhibition. The inhibitory effect was determined by comparing the presence or absence of a halo formed around the discs to that of the negative and positive controls with those of the experimental treatments [Table/Fig-2].
Effective percentage of inhibition of B. subtilis and M. luteus by ferrocenes concentrations relative to positive controls.
| Bacteria | Concentration I | Concentration II | Concentration III |
|---|
| B. subtilis |
| Fc-4Br-Ph | 44.5±1.9 | 41.6±1.3 | 43.8±1.5 |
| Fc-4Cl-Ph | 43.1±0.6 | 45±1.2 | 42.6±1.1 |
| Fc-4I-Ph | 46.6±2.2 | 43.3±1.1 | 45.5±1.5 |
| Fc-4F-Ph | 48.3±3.2 | - | - |
| M. luteus |
| Fc-Br | 42.48±1.4 | - | - |
| Fc-Cl | 44.68±1.2 | - | - |
Data shown in percentage ± standard error of the mean for each concentration. (-): no inhibition observed
Statistical Analysis
Descriptive statistical analysis was performed using IBM SPSS Statistics SPSS Version 21.0. (IBM Corp., NY, USA). Experiments were conducted in technical triplicates and with biological duplicates. When present, the diameter (mm) of the inhibition halo was measured and the values of the triplicates were averaged. The inhibitory effects of the ferrocenecarboxylates were calculated by dividing the average diameter of the halos of each compound by that of the control antibiotic and expressed as a percentage±standard error of the mean.
Results
The growth of B. subtilis was inhibited by all three concentrations of Fc-4Cl-Ph (10.16 μM, 5.08 μM, 2.54 μM); Fc-4Br-Ph (9.0 μM, 4.5 μM, 2.25 μM), Fc-4I-Ph (17.12 μM, 8.56 μM, 4.28 μM), and the highest concentration of Fc-4F-Ph (23.33 μM). Growth of M. luteus was inhibited by Fc-4Br-Ph (9.0 μM) and Fc-4Cl-Ph (10.16 μM) [Table/Fig-4]. Outcomes of treating nine bacterial isolates with ferrocenecarboxylates are listed in [Table/Fig-5].
Summary of organisms used and the observed results of inhibition by compound.
| Bacterium | Gram Reaction | Positive Control | Fc-Br | Fc-Cl | Fc-F | Fc-I | Fc-Ph | Fc-Py |
|---|
| S. aureus | + | Penicillin | - | - | - | - | - | - |
| E. coli | - | Kanamycin | - | - | - | - | - | - |
| M. luteus | + | Penicillin | +I | +I | - | - | - | - |
| P. aeruginosa | - | Kanamycin | - | -I, II, III NP | - | -I, II, III NP | - | - |
| S. marcescens | - | Kanamycin | - | - | - | - | N/A | - |
| K. pneumoniae | - | Kanamycin | - | - | - | - | N/A | - |
| B. subtilis | + | Kanamycin | +I, II, III | +I, II, III | +I | +I, II, III | N/A | - |
| P. vulgaris | - | Kanamycin | - | - | - | - | N/A | - |
| E. aerogenes | - | Kanamycin | - | - | - | - | N/A | - |
4-fluorophenyl ferrocenecarboxylate (Fc-4F-Ph), 4-chlorophenyl ferrocenecarboxylate (Fc-4Cl-Ph), 4-bromophenyl ferrocenecarboxylate (Fc-4Br-Ph), 4-iodophenyl ferrocenecarboxylate (Fc-4I-Ph), and their analog replacing the halide with a redox active specie 4-(1H-pyrrol-1-yl)-phenylferrocenecarboxylate (Fc-Py). Bacteria, gram-staining, antibiotic controls and concentrations (I: concentration I; II: concentration II; III: concentration III) used of ferrocenecarboxylates, with corresponding outcome.
(-): No inhibition observed; (+): Inhibition observed; NP: No pigmentation; N/A: not assayed
Discussion
The present microbiological study, determined the ability of ferrocenecarboxylates to inhibit bacterial growth by quantifying the halo of inhibition, measuring its diameter in mm. The observed antibacterial activity towards most of the tested Gram-positive bacteria can be attributed mainly to the structural differences between Gram-positive and Gram-negative bacteria in their cytoplasmic lipid membrane composition. Gram-positive bacterium possesses a thicker membrane with a diverse lipid profile that may increase ferrocene-membrane interactions [12]. Ferrocene compounds are lipophilic and allowing better penetration across the more lipophilic membrane present in the Gram-positive bacteria [13]. The organometallic redox active species Fc-Py did not showed any antibacterial activity against microorganisms tested, only ferrocene complexes with halogenated phenols demonstrated antibacterial properties [Table/Fig-4]. For example, fluorine has been shown to increase the biological effect of an organic compound. N-(2-chloro-5-nitrobenzylidene)-2-fluorobenzohydrazide which showed an effective antibacterial activity (against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Pseudomonas fluorescence) attributed to the electronegativity of the fluorine [14]. A patent submitted showed new nitromidazole derivatives with iodophenol to treat mycobacterial infections [15]. Bromophenols extract from marine algae showed growth inhibition of Botrytis cinerea, Pseudomonas fluorescence, Staphylococcus aureus, Vibrio cholera, and Proteus mirabilis [16]. 2-benzyl-4-chlorophenol is a well-known germicidal that recently was isolated as a natural product from Shewanella halifaxensis IRL548 [17]. All these active organic compounds have halogen atoms substituted on to the phenol side group, with the of exception of Fc-Py.
Previous studies, have demonstrated antibacterial, antimalarial, and antifungal properties of ferrocenes derivatised with large complex groups or substituted with known antimicrobials [4, 13-15, 17]. On the contrary, these compounds do not contain such substitutions, which may be susceptible to degradation and inhibition by microorganisms. It has been demonstrated that ferrocenoyl 17b-hydroxy-estra-1,3,5-(10)-trien-3-olate (ferrocene ester containing estradiol pendant group) exhibits anti-proliferative activity of with an IC50 ranging from 1.4-256 uM in the MCF-7 breast cancer cells and 1.8-500 uM in the MCF-10A non-malignant cell line [11]. These organometallic complexes demonstrated compatibility with biological systems; for this reason, this study explored bacterial growth, inhibition properties.
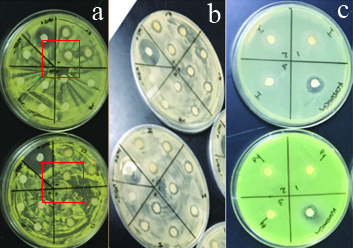
Although there was no observed inhibition of P. aeruginosa growth, Fe-4I-Ph and Fe-4Cl-Ph inhibited its pigmentation at all three tested concentrations [Table/Fig-6]. While it is unclear how these compounds interfered with the pigmentation of P. aeruginosa, mutations in pigmentation genes such as pyocyanin, have been linked to antibiotic resistance and resistance to phages [18]. Given the apparent importance of P. aeruginosa pigmentation the inhibition observed should be further studied.
Representative images of kirby-bauer test. Panel A) Micrococcus luteus; positive results indicated in red Fe-Br (9.00 μM) (top) and Fe-Cl (10.16 μM) (bottom) Panel B) Bacillus subtilis; positive results at all concentrations of Fe-Br (9.00 μM, 4.50 μM, and 2.25 μM) and Fe-I (17.12 μM, 8.56 μM, and 4.58 μM) Panel C) Pseudomonas aeruginosa; Fe-I interfering with the P. aeruginosa (top) and expected pigmentation (bottom), although there was no bacterial inhibition observed. *indicates positive antibiotic control and ** indicates vehicle control.
Limitation
This study demonstrates the proof of concept use of novel ferrocenecarboxylates as antibiotics for nine bacteria. Further studies should be conducted on other pathogenic organisms such as fungi to address plausible clinical applications. In addition, there may be differences in diffusion rate between these ferrocenecarboxylate compounds that may be considered in future studies specially in newly emerging resistant bacteria.
Conclusion
This study demonstrates that the anti-proliferative effects of 4-bromophenyl ferrocenecarboxylates (Fe-Br) are not exclusive to eukaryotes. Further studies should be conducted into the bioactive properties of these functionalised ferrocenecarboxylates to decipher pathways and modes of action. In addition, the observed effects were not broad spectrum across similar bacteria as were penicillin and kanamycin, indicating an underlying specificity. Where these compounds bind to and how this interaction interfere with growth has yet to be elucidated and could be the subject of future studies.
4-fluorophenyl ferrocenecarboxylate (Fc-4F-Ph), 4-chlorophenyl ferrocenecarboxylate (Fc-4Cl-Ph), 4-bromophenyl ferrocenecarboxylate (Fc-4Br-Ph), 4-iodophenyl ferrocenecarboxylate (Fc-4I-Ph), and their analog replacing the halide with a redox active specie 4-(1H-pyrrol-1-yl)-phenylferrocenecarboxylate (Fc-Py). Bacteria, gram-staining, antibiotic controls and concentrations (I: concentration I; II: concentration II; III: concentration III) used of ferrocenecarboxylates, with corresponding outcome.
(-): No inhibition observed; (+): Inhibition observed; NP: No pigmentation; N/A: not assayed
[1]. van der Horst MA, Schuurmans JM, Smid MC, Koenders BB, terKuile BH, De novo acquisition of resistance to three antibiotics by Escherichia coliMicrob Drug Resist 2011 17(2):141-47.10.1089/mdr.2010.010121235391 [Google Scholar] [CrossRef] [PubMed]
[2]. Webber MA, Buckner M, Redgrave LS, Ifill G, Mitchenall LA, Webb C, Quinolone-resistant gyrase mutants demonstrate decreased susceptibility to triclosanJ Antimicrob Chemother 2017 72(10):2755-63.10.1093/jac/dkx20129091182 [Google Scholar] [CrossRef] [PubMed]
[3]. Lewis K, Platforms for antibiotic discoveryNat Rev Drug Discov 2013 12(5):371-87.10.1038/nrd397523629505 [Google Scholar] [CrossRef] [PubMed]
[4]. Wenzel M, Patra M, Senges CH, Ott I, Stepanek JJ, Pinto A, Analysis of the mechanism of action of potent antibacterial hetero-tri-organometallic compounds: a structurally new class of antibioticsACS Chem Biol 2013 8(7):1442-50.10.1021/cb400084423578171 [Google Scholar] [CrossRef] [PubMed]
[5]. Van Staveren DR, Metzler-Nolte N, Bioorganometallic chemistry of ferroceneChem Rev 2004 (12):5931-86.10.1021/cr010151015584693 [Google Scholar] [CrossRef] [PubMed]
[6]. Fouda MF, Abd-Elzaher MM, Abdelsamaia RA, Labib AA, On the medicinal chemistry of ferroceneAppl Organomet Chem 2007 21(8):613-25.10.1002/aoc.1202 [Google Scholar] [CrossRef]
[7]. Braga SS, Silva AM, A new age for iron: antitumoral ferrocenesOrganometallics 2013 32(20):5626-39.10.1021/om400446y [Google Scholar] [CrossRef]
[8]. Gasser G, Ott I, Metzler-Nolte N, Organometallic anticancer compoundsJ Med Chem 2010 54(1):3-25.10.1021/jm100020w21077686 [Google Scholar] [CrossRef] [PubMed]
[9]. Meléndez E, Metallocenes as target specific drugs for cancer treatmentInorganica Chim Acta 2012 393:36-52.10.1016/j.ica.2012.06.00723180884 [Google Scholar] [CrossRef] [PubMed]
[10]. Wani WA, Jameel E, Baig U, Mumtazuddin S, Hun LT, Ferroquine and its derivatives: new generation of antimalarial agentsEur J Med Chem 2015 101:534-51.10.1016/j.ejmech.2015.07.00926188909 [Google Scholar] [CrossRef] [PubMed]
[11]. Vera JL, Rullán J, Santos N, Jiménez J, Rivera J, Santana A, Functionalized ferrocenes: the role of the para substituent on the phenoxy pendant groupJ Organomet Chem 2014 749:204-14.10.1016/j.jorganchem.2013.10.00227453588 [Google Scholar] [CrossRef] [PubMed]
[12]. Nawrocki KL, Crispell EK, McBride SM, Antimicrobial peptide resistance mechanisms of gram-positive bacteriaAntibiotics (Basel) 2014 3(4):461-92.10.3390/antibiotics304046125419466 [Google Scholar] [CrossRef] [PubMed]
[13]. García-Barrantes PM, Lamoureux GV, Pérez AL, García-Sánchez RN, Martínez AR, San Feliciano A, Synthesis and biological evaluation of novel ferrocene–naphthoquinones as antiplasmodial agentsEur J Med Chem 2013 70:548-57.10.1016/j.ejmech.2013.10.01124211630 [Google Scholar] [CrossRef] [PubMed]
[14]. Zhang M, Xian DM, Li HH, Zhang JC, You ZL, Synthesis and structures of halo-substituted aroylhydrazones with antimicrobial activityAust J Chem 2012 65(4):343-50.10.1071/CH11424 [Google Scholar] [CrossRef]
[15]. Kulangara VR, Atreya CR, inventors; Ge Healthcare Limited, assigneeNitroimidazole derivativesUnited States patent application US 13/700,187 2011 May 31 [Google Scholar]
[16]. Liu M, Wang G, Xiao L, Xu X, Liu X, Xu P, Bis (2, 3-dibromo-4, 5-dihydroxybenzyl) ether, a marine algae derived bromophenol, inhibits the growth of botrytis cinerea and interacts with DNA moleculesMar Drugs 2014 12(7):3838-51.10.3390/md1207383824979270 [Google Scholar] [CrossRef] [PubMed]
[17]. Moore SL, Berthomier L, Braganza CD, MacKichan JK, Ryan JL, Visnovsky G, Identification, library synthesis and anti-vibriosis activity of 2-benzyl-4-chlorophenol from cultures of the marine bacterium Shewanella halifaxensisBioorg Med Chem Lett 2016 26(13):3086-88.10.1016/j.bmcl.2016.05.00227185331 [Google Scholar] [CrossRef] [PubMed]
[18]. Le S, Yao X, Lu S, Tan Y, Rao X, Li M, Chromosomal DNA deletion confers phage resistance to Pseudomonas aeruginosaSci Rep 2014 4:473810.1038/srep0473824770387 [Google Scholar] [CrossRef] [PubMed]